Radioactive nuclei emit three types of radiations
Physicists have called the three types of radiations emitted by nuclei, alpha, beta and gamma, the three first letters of the greek alphabet.
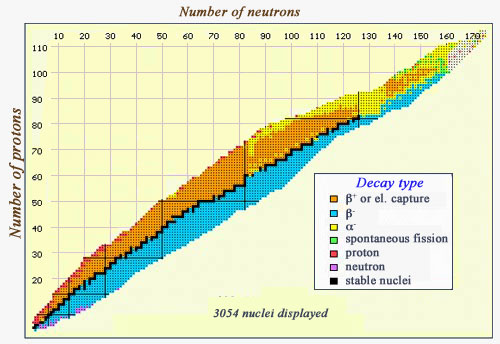
Map of decay modes
This map of the various nuclei is coloured with regards to the types of decay they undergo. Stable nuclei, along the “stability” line, are in black. The beta-emitters can be found on either side of this curve – beta negatives (in blue) occur in neutron-heavy nuclei, whereas beta-positives (in orange) occur in proton-heavy nuclei. At the right-hand side, the line of stability is replaced by a zone where alpha-emitters (in yellow) predominate. One can also see some exceedingly heavy particles (very far from the line of stability) which undergo spontaneous fission (in green), and a few nuclei which emit either protons (in red) or neutrons (in purple)
IN2P3/NuBase
This naming convention of the three types of radiation has been in use since their discovery, and still applies today. The ancient greek alphabet was familar to physicists nourished by classical culture.
Alpha radiation is the name for the emission of an alpha particle in fact an helium nucleus beta radiation is the emission of electrons or positrons, and gamma radiation is the term used for the emission of energetic photons.
When uranium salts were found in 1896 to produce unknown emissions, two types of radiation, X-rays and cathode rays, have been just discovered. At that time, nuclei, electrons and photons were unknown. It would take decades before the origins of all these rays were properly understood, but a few years to identify their nature. Incidentally cathode rays and X-rays were found to be electrons and photons like beta and gamma radiations.
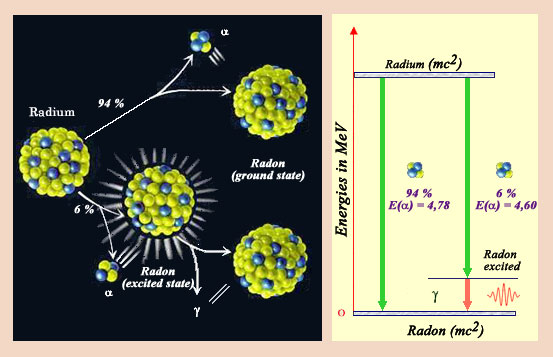
Disintegration diagram
This nucleus of radium-226, the one discovered by Marie Curie, decays directly in 93 % of the cases in a nucleus of radon by emitting an alpha particle. However in 7 % of the cases, the nucleus is left in an excited state. Very rapidly, the excited radon nucleus get rid of its excess of energy, by emitting a gamma ray. Physicists represent the various decay modes of a nucleus by decay charts similar to the one represented on the right. The emission of gamma very often accompanies the transformations of nuclei.
© IN2P3
Alpha, beta and gamma decay are a result of the three fundamental forces working in the nucleus – the ‘strong’ force, the ‘weak’ force and the ‘electromagnetic’ force. In all three cases, the emission of radiation increases the nucleus stability, by adjusting its proton/neutron ratio.
In the case of alpha radiation, the nucleus attempts to find stability by emitting an ‘alpha particle’ – identical to a helium nucleus (two protons and two neutrons).
Beta radiation involves the transformation of a neutron into a proton through the emission of an electron, or the reverse process, the transformation of a proton into a neutron through the emission of a positron (similar to an electron, but with a positive charge).
Gamma radiation is simply a loss of energy by the nucleus, a desexcitation ; much like an emission of light or X-rays by energetic atoms. Alpha and beta decays almost always leave the nucleus in an excited state. Gamma emission brings the nucleus down to a more stable energetic state.
Alpha and beta decays are often difficult to occur. They can be very slow processes. The lifetimes of some radioactive nuclei are long for the clocks of the infinitely small. They can also be long for us. The lifetimes of natural radioactive alpha emitters such as uranium or thorium can extend to several billions of years.
These emissions change the composition of the nucleus, therefore the nature of the atom. Alpha and beta radioactivities do not transform lead into gold, but transmute matter like other nuclear reactions do.
Articles on the subject « Alpha Beta Gamma rays »
Beta (β) radioactivity
How Nature corrects an excess of protons or neutrons Beta (β) radioactivity was first observed in[...]
Beta spectrum
Beta electrons do not have a unique characteristic energy Three particles, the recoil nucleus, th[...]
Electron Capture
A minor mode … competing with positron emission Electron capture is a comparatively minor d[...]
Positron
The positive electron, the first ambassador of antimatter The positron was discovered in 1933 by [...]
The electron-neutrino
An electron that would have lost its electric charge The easiest way to conceive a neutrino is to[...]
Muons
An heavy electron abundant in cosmic rays Muons were first observed in 1936 by the Americans phys[...]
Radioactivity Gamma (γ)
How nuclei get rid of excess energy It was in 1900 that the French physicist Paul Villard first f[...]
Nuclear Desexcitations
Gamma are the light emitted by atomic nuclei A french proverb used to say “all roads go to [...]
Internal Conversion
When a gamma expells an atomic electron and is absorbed Internal conversion is a nucleus desexcit[...]
Nuclear Transmutations
The ancient dream of the alchemists… The alchemists of the Middle Ages and the Renaissance [...]