Cross section or the interaction probability of a particle
Cross section is the name given by physicists to the interaction probability of a particle. In the fields of radioactivity and nuclear energy, the particles concerned are gamma rays, neutrons and to a lesser extent beta electrons in the case of Bremsstrahlung . The interaction targets are nuclei.
A gamma ray snatches an electron from a atomic deep layer ; a neutron is captured by a nucleus: another neutron triggers the fission of a uranium nucleus … The projectiles and their targets alike are extremely tiny objects. How can one measure the likelihood that they interact ? Physicists relate this interaction probability to an apparent size of the target : they call it cross section.
Neutron cross section of a target nucleus
Nuclear forces felt by a neutron approaching a nucleus are short range. The apparent size seen by the neutron approaching a nucleus is in general of the order of its geometrical section, ie very small. However, when the neutron is slow or at some particular “resonant” energies, this apparent size – cross section – may become much larger than the nucleus geometrical section.
© IN2P3
As its name suggests, a cross section represents a surface. The unit used is the barn, a unit adapted to the scale of the extremely small areas attributed to nuclei taken as targets : 1 barn equals 0.000 000 000 000 000 000 000 001 cm2 (10 to the power -24). The “section” of a proton or neutron assimilated to a tiny compact ball is 0.07 barn. So, a barn roughly corresponds to the section of middle size nucleus.
Cross section are among the key data of elementary particles physics, for instance, the cross sections of low energy neutrinos coming from the sun. They are so small (of the order of a billionth of a millionth of a barn) that on 10 billion of these neutrinos passing through the Earth only one will interact with the earth atoms during their journey.
In the areas of radiological protection and reactor operations, the relevant cross sections are those of neutrons and gamma. Particles carrying an electric charge, such as alpha particles or beta electrons, interact owing to this charge with thousands of atoimc electrons as they travel through matter. On the contrary, neutral particles, like neutrons and gamma, interact with a unique target : a neutron with a nucleus; a gamma usually with an electron belonging to the electronic cloud of an atom.
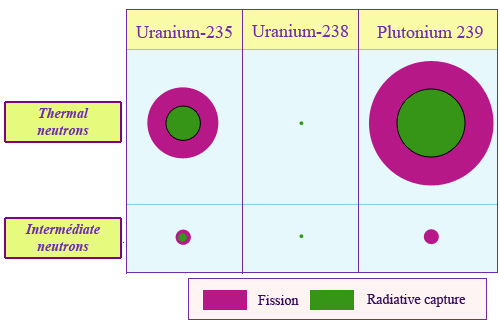
Global and partial cross sections
The apparent sizes as seen by neutron of three heavy nuclei, uranium-235, uranium-238 and plutonium, are represented on the figure by shooting targets. Target areas represent total cross sections, central parts radiative neutron captures, red band captures followed by fission. One can see that in the two energy ranges of neutrons called thermal and intermediate, the global and partial cross-sections areas are extremely different for the three nuclei. : capture and fission uranium-238 cross sections are much smaller than that of uranium-235 and plutonium-239.
© IN2P3
Finally, one should precise the interaction result , define the final state ! To each final state corresponds a partial cross section , which is a part of the total cross section which includes all final states.
A neutron may simply bounce off an atomic nucleus or be captured by it. The capture can be followed by a gamma emission, cause a nuclear reaction, for example a nuclear fission. The corresponding cross sections then would be referred to elastic collision, radiative capture, fission, etc …
A gamma can be absorbed by an electron that it pulls from the atom (photoelectric effect), it can simply collide with an electron (Compton effect) or if its energy exceeds 1.020 MeV it can also interact with the nucleus to create an electron and a positron (pair production).
In the case of neutrons, cross sections varies considerably fron one nucleus to another. One example is the process of radiative capture. Depending on whether the community of neutrons and protons that make up the target nucleus tends to reject the intruder – an extra neutron – or welcomed with open arms, the capture will be difficult or easy. This is explains for instance why the cross section capture of slow neutrons is minimal for uranium-238 but important for uranium-235.
Cross sections vary also greatly with the incident particle energy. An interaction results to a great extent from an “exchange” between the two particles involved. This is the case for the photoelectric and Compton effects with gamma. For neutrons, despite the short-range character of nuclear forces, a distant exchange could occur if the neutron is slow and spends enough time near the nucleus. A fast neutron would have less time to remotely. Cross sections would diminish with speed.
The neutrons cross sections can become very large if the neutron resonates with the nucleus: ie if it brings exactly the amount of energy required for the formation of an excited state of the nucleus. Cadmium, a rare metal used in neutron shielding, has an isotope – cadmium-113 – whose capture cross section reaches 20,600 barns for a very low neutron energy (0.2 electronvolt). Cadmium is used for neutron shielding.
The record is held by two isotopes of gadolinium, a rare earth chemical, elements, present only in trace amounts in nature : gadolinium 155 (61,000 barns) and gadolinium 157 (254,000 barns).
NEXT : Slow neutrons
NEXT : Fast neutrons
Other articles on the subject « Radiations effects in matter »
Charged Particle Effects
A gradual loss and transfer of energy Alpha rays, fission products ; heavy, slow and ionizing par[...]
Alpha Rays in Matter
An atomic bulldozer, strongly ionizing along a very short path Alpha particles are simultaneously[...]
Beta Rays in Matter
Light electrons : a chaotic journey through matter Beta electrons and positrons have equal and op[...]
Bremsstrahlung
A relativistic phenomenon that applies to electrons and positrons… The phenomenon of bremss[...]
Neutral Particle Effects
Energy transfer by proxy… Neutral particles that are of interest in the field of radioactiv[...]
Cherenkov Effect
When an electron goes faster than light in air and water … The Cherenkov effect occurs when[...]
Gamma Rays in Matter
Gamma can be attenuated but never fully stopped The neutral gamma rays leave very different effec[...]
Photoelectric Effect
The most effective mechanism of photon absorption The photoelectric effect is the phenomenon that[...]
Compton Effect
Photons as projectiles and electrons as targets The Compton effect is the name given by physicist[...]
Macroscopic Effects
Effects on inert or organic matter The ionisation of atoms surrounding the trajectory of an alpha[...]