The way radioactivity works and its origins
The impressive range of half-lives that exist, extending from fractions of a second to billions of years, gives us valuable clues to understanding how complex the origins of radioactivity are. Short half-lives indicate a radioactive decay that takes place easily, while a longer half-life signifies that a complicated and difficult process is taking place. These processes involve interplay between the three nuclear interactions: the strong force, the weak force, and the electromagnetic force.
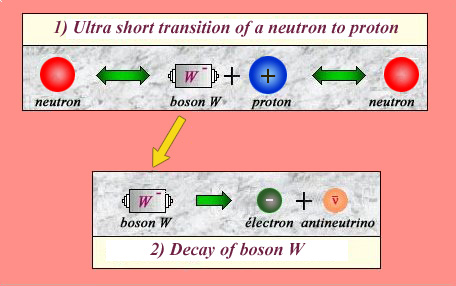
Neutron decay :An example of weak forces
Quantum mechanics allows a particle to undergo brief transformations, provided it returns to its original state. For instance, a neutron can briefly transform itself as a proton accompanied by a negatively-charged object called ‘W boson’. During the extremely short duration of this transformation, the W boson may decay into an electron and an antineutrino. The probability of the intermediary stage combined with the probability of the W boson decaying is so low that the neutron-proton decays are found to be slow. For this reason the interaction has been dubbed ‘weak’. The W boson was observed as a particle for the first time at CERN in 1983.
© IN2P3
The most important factor in any decay is the amount of energy available. The greater the energy released, the faster and easier the decay will be. There are, however, other conservation laws that need to be obeyed, which may slow down some nuclear transformations, and forbid others.
The competition between the attractive and repulsive forces in the nucleus is one of the factors which slow down alpha decay. When an alpha particle is in the process of being emitted from an heavy nucleus, it experiences an attractive force from the protons and neutrons in the nucleus it has just left. In order to escape, the alpha particle must find a way to jump the energy barrier the attraction has set.
Beta decay is a more complicated procedure as it requires the transformation of one type of nucleon (proton or neutron) into the other, a process accompanied by the formation of electrons, positrons and neutrinos. This creation of particles which do not exist in the nucleus is achieved by a force negligible in strength when compared to the ‘strong’ nuclear forces or the repulsive electric ones : the weak force. This aptly-named ‘weak’ force plays an important role in the structure of the nucleus, by allowing transformations to take place which increase the nucleus’s stability.
Gamma emissions can not be assimilated to a nucleus decay. They correspond to a simple restructuring of the nucleus in an excited state and are due to the electromagnetic forces present in it. They follow often alpha, beta decays or neutron captures and take place almost immediately; with rare exceptions such as technetium 99. This decay is an example of the slowing down of some nuclear transformations.
The nucleus of technetium 99 is the product of the beta decay of a parent nucleus, the molybdenum 99. The technetium 99 is left into an excited state. Excited nuclei return to their normal state – the ground state – by emitting a gamma photon. Ordinarily this release of energy would happen instantaneously, but in the case of technetium 99 the process takes six hours because the transition from the excited to the ground state is almost forbidden. This technetium may look a curiosity of physics, but its 6 hours lifetime makes it a pure gamma emitter, an ideal tool for radiodiagnostics in medicine.
Learn more :
Other articles on the subject « Radioactive nuclei »
Map of Nuclei
Map of stable and unstable nuclei The progress made in our understanding of the subatomic world o[...]
Stability Valley
Beta decay : nuclei getting slimmer to achieve stability The nucleus mass is related to its inter[...]
Nuclear Forces
Three nuclear forces and their hierarchy Three types of force act alongside each other inside a n[...]
α decay : tunnel effect
Particle and wave: an effect of quantum mechanics The great age of uranium and thorium nuclei tha[...]
Alphas with gammas
Gamma rarely accompanies alpha decays It is surprising that one can hold with hands a sample of p[...]
β decay : weak forces
The forces which allow a nucleus to emit beta electrons Beta decay (β) and electronic capture cha[...]
Weak Forces
A special and fascinating fundamental interaction A third force is at work in the nucleus next to[...]
The Nucleosynthesis
Primordial and stellar nucleosynthesis The Universe hasn’t always existed, It was born a li[...]
Nucleosynthesis (continued)
Mechanisms of atomic nuclei formation Most of the nuclei of atoms that make up our daily life wer[...]