Track down and understand phenomena with radioactive atoms
We owe our knowledge of isotopes to the pioneering work done by the Austro-Hungarian physicist Georg von Hevesy, and his contemporaries Frederick Soddy and Kasimir Fajans. Von Hevesy was also the first to develop the ‘tracer method’, a technique he finally perfected in 1923. For two years he had failed to extract by chemical methods radium-D (given the name because it was at the time the unknown fourth descendant of radium) from lead. This isotope is now known to be lead 210, a radioactive element with a half-life of 22.3 years. The reason for von Hevesy’s failure was the impossibility of chemically distinguishing between lead 210 and its stable isotopes
Von Hevesy reasoned that since chemical reactions had been of no use in his attempts at extracting radium-D from lead, no biological process could achieve the separation either. He sprinkled plants with water containing lead (in turn containing radium-D), and was thus able to follow the movement of lead through the plants by measuring the radioactivity of the roots, the stem and, eventually, the leaves.
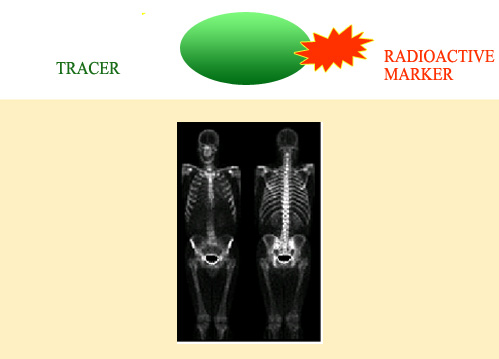
Tracers and markers in medicine
The tracers used in medicine are complex organic molecules specifically chosen for their affinity for the organ under examination. These molecules are ‘marked’ by the presence of a radioactive nucleus, and are often injected intravenously. On these skeletal scans, one can see the areas where a diphosphate marked with technetium 99 has built up, indicating the possible presence of metastasis. This particular marker is very used in gamma scintigraphy.
© M.O.Habert/Pitié-Salpétrière
If order to trace the journey of a specific molecule, one can replace a stable atom with a radioactive isotope (such as replacing hydrogen with tritium), and follow the trail left by the radiation. This process is known as radioactive ‘marking’ rather than ‘tracing’, as the isotope in question is merely playing the part of an identifying sticker.
For instance, a radioisotope like cobalt-60 would be called a tracer if used to trace the behaviour of cobalt in a chemical process. On the contrary, we would talk of marking with cobalt 60 in a metallurgical process whereby steel balls are coated with this radioisotope so as to highlight the flow of these steel balls. The distinction between markers and tracers, however, is confusing for the non-specialist, and one is often taken for the other.
Thanks to radioactive isotopes it is now possible to follow the process of a specific chemical or particle without altering the structural, chemical or hydrodynamical properties of the substance. In the same way, it has been possible to monitor the radioactive fall out and the residual pollution of the nuclear tests and of the Chernobyl accident.
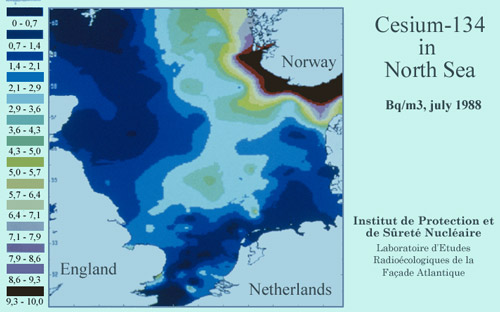
Map of césium marine pollution after Tchernobyl
This map shows the distribution in 1988 of the cesium-134 activity in the North Sea, two years after the Chernobyl accident. This radioactive cesium isotope is a beta and gamma emitter and has a two-years half-life. Cesium-134 was chosen here to monitor the radioactive pollution of cesium in the Nortn Sea diffused by water currents. The south coast of Norway was the most affected.
© CEA/IRSN
The extreme sensitivity of radioactivity detectors means that infinitesimal amounts of radioactive tracers can be detected. One uses generally gamma ray emitters, since gamma are penetrating, able to pass through large amounts of matter, and more easy to detect. This last restriction is hardly a limiting factor, as most radoelements emit gamma rays. One further advantage is that many radioactive tracers have short half-lives and disappear quickly and completely from the environment in which they were introduced..
Tracers commonly used in biology, to explore natural processes, and in medicine, where they have become a vital part of many scans and diagnostic tests. They also provide us with a method of studying the workings of the environment (sea currents, groundwater tables), or industrial techniques. Radioactive tracing is a helpful tool in searching for leaks, measuring flows, and calculating the propagation of pollutants produced.