A fission product with properties close to calcium
Strontium-90 is with cesium-137 a major radioactive product of nuclear fission. After the explosion of an atomic bomb or within a nuclear reactor, it is abundant: 5.8% of uranium-235 fissions produces this radioelement. There was mention of strontium-90 during testing atomic bombs in the atmosphere of the 1960s. It was also widespread in accidents like those of the Techa River, Chernobyl and recently Fukushima.
Chemically, strontium is close to calcium in the Mendeleev classification. Like calcium, when absorbed, it is preferably fixed in the bone mass. It emits only beta radiation with a short range, which makes it harmful if swallowed or inhaled. In this case, it may be the source of bone cancers and leukaemias if the spinal cord is involved.
In food, milk and calcium-rich cheese favour strontium. At sea, strontium will be fixed more on oyster and mussels, crab and shrimp shells, fish bones and scales than in fish flesh. Strontium remains generally at low levels in aquatic organisms.
Strontium-90 does not emit gamma rays. Owing to the absence of very characteristic energy rays which would sign its presence, strontium-90 presence is difficult to identify. This absence of gamma rays also means reduced external exposures. One can tread without excessive risks ground contaminated by strontium-90 …
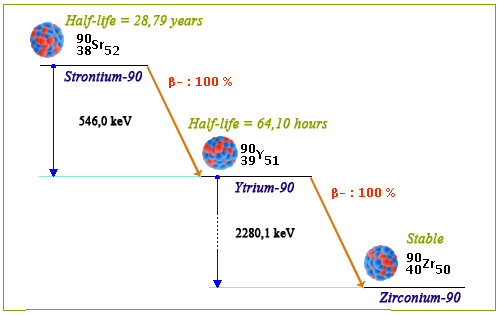
Strontium-90 decay diagram
The strontium-90 decay diagram displays two main features. First, it happens to be a cascade decay. Strontium-90 decays into unstable Ytrium-90 that later decays into stable zirconium-90. The Ytrium lifetime is much shorter than that of Strontium-90 (respectively 64 hours and 29-years radioactive half-lives). Moreover, the two successive decays are almost 100% beta.. There is no accompanying gamma rays allowing to easily identify the decays and the emitted beta rays, though energetic, are not penetrating.
© IN2P3 (Source BNL – Nudat2)
Strontium-90 is less dispersed than cesium after reactor accidents because it is significantly less volatile, as has been observed in Chernobyl. Mobility and transfer into the environment however remain poorly understood.
Strontium transport by surface water and groundwater has been studied in the Chernobyl zone where large amounts of radioactive materials were found buried in a sand layer of 5 to 7 meters thick lying in a stagnant phreatic table. On the vertical, strontium 90 has contaminated ground water at around 10 kilobecquerels per liter (kBq / l). Tens of meters away, this contamination falls to about 0.1 kBq / l. In this situation, the migration of strontium-90 with water appeared to have been slow.
In Fukushima The strontium 90 issue has come up in the fall of 2013 near Fukushima after leaks of contaminated water that was stored in tanks. While a cesium decontamination of water was in place since 2011, that of strontium was not yet operational. Strontium-90 was present in water stored on the site, part of which was spilled mainly in March-April 2011 in the coastal zone.
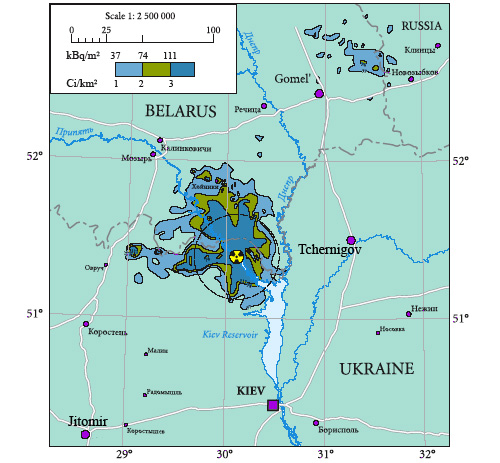
Strontium-90 deposits around Chernobyl
Distribution in December 1989 of strontium-90 deposits 3 years after the Chernobyl accident. The presence of strontium being much harder to detect than that of cesium-137, ground contamination maps like this are rare. The strontium ground contamination appears significantly lower than that of cesium-137 and more concentrated around the accident. Radioactivity being only beta rays, ground contamination does not produce significant external exposure.
© Chernobyl Atlas
The lifespan of strontium-90 is long on a human scale with a radioactive half-life of 28.79 years, close to that of cesium-137. In a first step it decays into Ytrium-90, which being unstable itself becomes Zirconium-90 after a few tens of hours.
The energy available in the two decays in cascade is high, 2736 keV, the two beta electrons emitted taking on average half this amount. They dissipate locally their energy as heat after a short course in matter. Thereby, strontium-90 radiates a relatively large amount of heat : 0.91 watt per gram.
This energy release has led to the use of strontium-90 in the former Soviet Union for thermoelectric generators called RTG for supplying energy and electricity to a thousand beacons and unmanned lighthouses in the remote Siberian north. Such sources were much cheaper than plutonium-238 sources embarked for distant space missions of NASA.
The substantial heat released by strontium decays intervenes in the management of high level radioactive waste. When a vitrified waste package is produced at the La Hague plant, a quarter of the heat released – 500 watts – is due to strontium decay. This heat release requires many years of cooling before burying these waste packages in a deep geological repository. However at the time scale of such geological storages, the radioactivity and the heat release by strontium-90 decline by large amounts : they are divided by 11 within a century, by 1380 in three centuries..
Other articles on the subject « Main Radioactive Nuclei »
Uranium 238 and 235
A radioactive and strategic element The uranium atom is the heaviest atom present in the natural [...]
Plutonium 239
Plutonium 239: an artificial fissile nucleus, highly sought-after and feared Plutonium, the ninet[...]
Plutonium Properties
A transuranic element with long-lived radiotoxic isotopes Plutonium is a very dense metal, radioa[...]
Radium
The radioactive nucleus that made History Radium is an extremely rare element that was first disc[...]
Carbon-14
A by-product of cosmic rays The nucleus of carbon 14 contains 6 protons and 8 neutrons, instead o[...]
Potassium-40
A curiosity of Nature and a very long lived beta emitter Potassium 40 is a radioisotope found in [...]
Iodine 131
Radioactive iodine : A dangerous and short lived fission product Iodine 131 is a radioisotope wit[...]
Tritium
A radioactive isotope of hydrogen Tritium is a beta-emitting radioactive isotope of hydrogen. Its[...]
Caesium 137
A legacy of atmospheric nuclear bomb tests and accidents Caesium 137 is a radioactive element wit[...]
Technetium 99
A pure gamma emitter widely used in nuclear medicine Of all the atoms below uranium in Mendeleyev[...]