A curiosity of Nature and a very long lived beta emitter

Potassium-40 decays into Argon 40, a gas held prisoner by lava
The potassium-argon method is used to date lava flows whose age is between a million and a billion years. When an atom of potassium 40 decays into argon 40, the argon atom produced is trapped by the crystalline structure of the lava. It can only escape when the rock is in its molten state, and so the amount of fossilized argon present in lava allows scientists to date the age of the solidification.
© DR
Potassium 40 is a radioisotope found in trace amounts in natural potassium, is at the origin of more than half of the human body activity: undergoing between 4 and 5,000 decays every second for an 80kg man. Along with uranium and thorium, potassium contributes to the natural radioactivity of rocks and hence to the Earth heat.
This isotope makes up one ten thousandth of the potassium found naturally. In terms of atomic weight, it is located between two more stables and far more abundants isotopes (potassium 39 and potassium 41) that make up 93.25% and 6.73% of the Earth total potassium supply. With a radioactive half-life of 1,251 billion years, potassium 40 existed in the remnants of dead stars whose agglomeration has led to the Solar System with its planets.
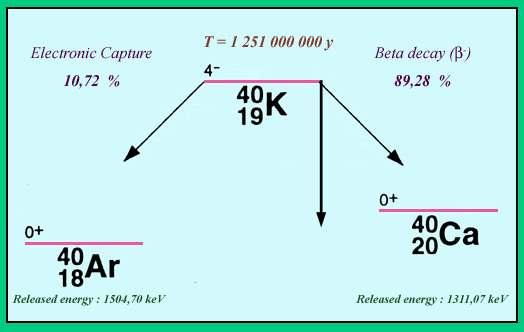
The two decay channels of potassium 40 (K-40)
The decay scheme of potassium-40 is unusual. Its mass energy is above these of its two neighbours in the family of atoms with 40 nucleons : Argon-40 with one proton less and calcium-40 with one proton more. Potassium-40 has two decay channels open. The beta-minus decay channel leading to calcium-40 is by far the most frequent. Decays leading to argon-40 by electronic capture occurs at a 11 % rate. Remarkable also is the K-40 very long half-life of 1,251 billion years, exceptional for a beta decay. This is explained by a large jump in the internal nucleus rotation (or spin) during the decay, which almost forbids the transition, therefore making it extremely slow.
© IN2P3
Potassium 40 has the unusual property of decaying into two different nuclei: in 89% of cases beta-negative decay will lead to calcium 40, while 11% of the decays argon 40 will be formed by electron capture followed by gamma emission at an energy of 1.46 MeV.
This 1.46 MeV gamma ray is important, as it allows us to identify when potassium 40 decays. The beta electrons leading to calcium, however, are not accompanied by gamma rays, have no characteristic energies and rarely make it out of the rocks or bodies that contain potassium 40.
Beta-minus decay indicates a nucleus with too many neutrons, electron capture a nucleus with too many protons. How can potassium 40 simultaneously have too many of both? The answer reveals one of the peculiarities of the nuclear forces.
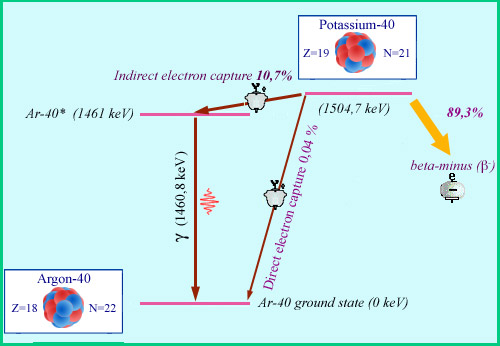
From potassium 40 to argon 40
The electron capture which causes potassium 40 to transform into argon 40 in its ground state takes place in only 0.04% of cases. Far more frequently (10.68% of the time), an indirect capture leads to an excited argon atom which returns to its ground state by emitting a 1.46 MeV gamma ray. Without this characteristic gamma ray, it would be impossible to detect and identify the decay of potassium 40. The beta electrons of the decay into calcium 40 (89.3% of the time) are not accompanied by gamma rays, and are generally absorbed into the medium.
© IN2P3
Stable nuclei sit at the bottom of a so-called ‘valley of stability’, a concept that helps determine whether a nucleus is radioactive or not. Potassium 40 should be at the bottom of this valley and should be the most stable of the nuclei containing 40 nucleons. Its mass energy (or internal energy), however, is actually greater than either of its neighbours – calcium 40 and argon 40. This difference is enough to make potassium 40 unstable. The reason for this is that protons, like neutrons, like to exist in pairs in a nucleus. Potassium 40 contains odd numbers of both – 19 protons and 21 neutrons. As a result it has one bachelor proton and one bachelor neutron. In both argon 40 and calcium 40, however, the number of protons and neutrons are even, granting them that extra stability.
The very slow decay of potassium 40 into argon are highly useful for dating rocks, such as lava, whose age is between a million and a billion years. The decay of potassium into argon produces a gaseous atom which is trapped at the time of the crystallization of lava. The atom can escape when the lava is still liquid, but not after solidification. At that moment, the rock contains a certain amount of potassium but no argon. With time and the potassium 40 disintegrations, the gaseous argon atoms accumulate very slowly in the lava where they are trapped. Measuring the amount of argon 40 formed since the solidification of the lava allows for an accurate measure of the rock age.
Other articles on the subject « Main Radioactive Nuclei »
Uranium 238 and 235
A radioactive and strategic element The uranium atom is the heaviest atom present in the natural [...]
Plutonium 239
Plutonium 239: an artificial fissile nucleus, highly sought-after and feared Plutonium, the ninet[...]
Plutonium Properties
A transuranic element with long-lived radiotoxic isotopes Plutonium is a very dense metal, radioa[...]
Radium
The radioactive nucleus that made History Radium is an extremely rare element that was first disc[...]
Carbon-14
A by-product of cosmic rays The nucleus of carbon 14 contains 6 protons and 8 neutrons, instead o[...]
Iodine 131
Radioactive iodine : A dangerous and short lived fission product Iodine 131 is a radioisotope wit[...]
Tritium
A radioactive isotope of hydrogen Tritium is a beta-emitting radioactive isotope of hydrogen. Its[...]
Caesium 137
A legacy of atmospheric nuclear bomb tests and accidents Caesium 137 is a radioactive element wit[...]
Strontium-90
A fission product with properties close to calcium Strontium-90 is with cesium-137 a major radioa[...]
Technetium 99
A pure gamma emitter widely used in nuclear medicine Of all the atoms below uranium in Mendeleyev[...]