Using X-rays and radioactivity to destroy cancerous cells
Soon after his 1896 discovery of radioactivity, Henri Becquerel noticed that the small amount of radium he always carried in his jacket pocket was causing a rash on his skin. In 1901, under similar circumstances, his colleague Pierre Curie noticed an identical rash appearing on his arm. Instances such as these prompted a sudden interest in possible medical uses of radioactivity – specifically as a tool to treat tumours. After Pierre’s death, Marie Curie and Claudius Régaud combined physics, chemistry and medicine to become pioneers in a completely new kind of therapy.
Despite their enormous importance in the field, the Curie were not the first to venture into the medical applications of radiations: the first use of X-rays in medicine came only weeks after their original discovery in 1895. As soon as 1901, Dr Danlos of the Saint Louis hospital in Paris was already using exposure to radium to help treat a variety of skin problems.
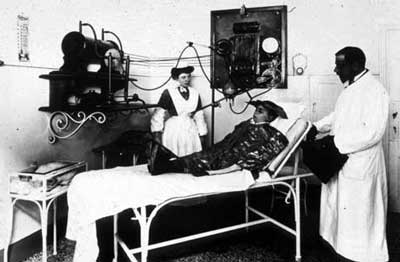
Radiotherapy in 1925
The installation of a radiotherapy apparatus in Italy in 1925. The young man on the bed will be subjected to X-rays coming from the machine to the left of the picture to cure tinea on his head. As can be seen, the X-rays will be spread throughout the entire room. At that time, virtually no precautions were taken. There were no techniques available to localise the incoming rays.
© Institut Curie
Since then, hundreds of thousands of cancers have been treated by exposure to radium and its successors: iridium 192 and caesium 137. The radiation emitted by these particular isotopes has proven useful in treating a host of different diseases.
Within one hundred years, radiation therapy (using virtually all types of radiations) has developed into a highly sophisticated medical technique. Used in 50% of all cancer treatments, it has the ability to integrate seamlessly into current procedures, and is used side-by-side with surgery and chemotherapy.
Modern radiation therapy
Radiotherapy today includes all cancer treatments based on ionizing radiation, without distinguishing the origin of this radiation, atomic (X rays), nuclear or particle produced by accelerators.
It is not possible to distinguish an X-ray photon from a gamma having the same energy, nor an atomic electron from a beta electron expelled by a nucleus. Biological and therapeutic effects are the same, whatever the origin of the radiation or particle. However, the techniques vary depending on the radiation source.
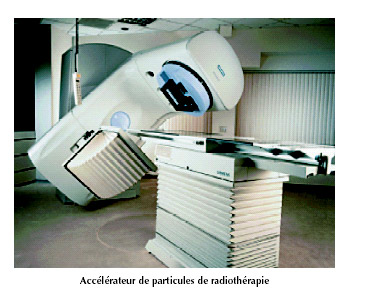
Small accelerators used in radiotherapy
This linear accelerator shows shows the long way radiotherapy has come. These technicals equipments are now being used, gradually replacing the old cobalt 60-based treatment in most hospitals. The great advantage of these machines is their ability to direct the beams with higher degrees of accuracy.
© ASN 2003 Report
X or gamma rays go through a body part before reaching their target, for example a deep tumor. In a developed country like France, over a hundred thousand cancer patients a year receive radiation therapy. Nowadays, gamma rays have replaced X-Rays. Gamma rays beams are generated by small accelerators. They replace the gamma rays produced by radioactive cobalt-60 decays in “cobalt-bombs”
The impressive advances made over the last century have led for the emergence of “conformal therapy”: a procedure which allow to target the affected areas with accuracy. Using complex 3-D scans of the tumour and the surrounding organs, the incoming radiation can be adapted to fit the exact shape and size of the tumour, concentrate the energy deposition in the target, minimizing around the dose absorbed by healthy cells, therefore reducing the risks posed by radiation therapy.
These techniques of radiotherapy are combined today by oncologists with chemotherapy and surgery to define, the most effective therapeutic strategy in each case.

Proton therapy for eye melanoma
Treatment of an eye melanoma in the Orsay Proton Therapy Centre. ‘Beams’ of particles (protons, in this case) are used as a replacement for radioactive sources in a variety of radiation treatments. When dealing with an uveal melanoma (the name for tumours found in the retina), it is crucial to avoid unnecessarily exposing the brain – mere millimetres away – to radiation. Consequently, the high-precision proton beams are preferred.
© Centre de Protonthérapie d’Orsay
In metabolic therapy, it is now possible to have the benefit of all the advances made in biology and pathophysiology to “associate” a radioactive substance to a metabolism or to a metabolic product, which will then, by-itself, locate and go to the tumour area or nearby.
Brachytherapies are a much less frequent kind of therapies. Brachytherapies were first proposed by Marie Curie and Claudius Regaud with radium needles.The principle is to fix radioelements on or near a tumour. The radioelements should emit beta rays or low energy gamma rays with a range of a few centimeters. They should also have a short lifetime and disappear after a few weeks or months. Examples are the treatment of hyperthyroidism and thyroid cancers by iodine-131 and brachytherapies of prostate using iodine-125 or palladium-123. (like for example capsules of iodine-125 in cancer of the prostate, directly inserted into the tumour tissue)
More rarely, particle beams provided by an accelerator are used to shell malignant tissues. These therapies are called hadrontherapies or proton therapies in the case of protons. The beam energy is tuned so that the particles stop in these malignant tissues where they cause maximum damage. For instance, proton therapies are used today in the treatment of eye melanoma.
An effective therapy relies on the application of appropriate doses and the ability to follow a patient’s progress after the exposure. Improved detection capabilities and data storage facilities have contributed to making radiotherapy an indispensable part of responses to cancers.
It must not be forgotten also that radiation treatment carries exposure-related risks: risks that doctors and radiologists have learnt to control and limit.
Articles on the subject « Radiation Therapy »
Radiotherapies
X-rays but no radioactivity Radiotherapy today denotes all cancer treatments based on ionizing ra[...]
Metabolic Therapies
Therapeutic Radioisotopes Applications of radioisotopes in nuclear medicine are not limited to sc[...]
Brachytherapies
The oldest nuclear therapy modernized today Brachytherapies (or Curietherapies) are the oldest th[...]
Prostate Brachytherapy
An Efficient destruction of cancerous cells Since the 2000s, particularly in the United States an[...]
Protontherapies
Proton therapy: an advanced and precise radiotherapy Proton therapy involves treating tumors with[...]
Secondary Risks
Alternative: exposure to radiation or let the cancer evolve… For over 20 years, there has b[...]