The decay rate and the rate of radiation emitted
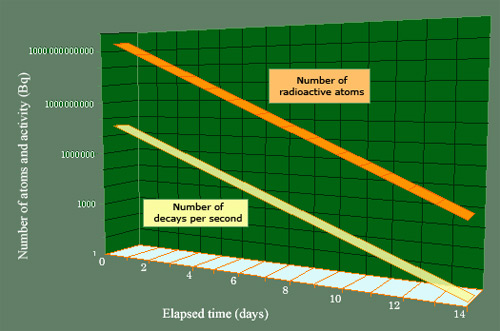
Parallel decrease of the number of nuclei and activity
The activity and the mass of a radioactive body diminish at the same rate (the mass has been expressed in terms of the number of atoms in order for it to be placed on the same scale). The radioactive body chosen is iodine 123, and the initial activity (of 1 millicurie) is equivalent to that of a thyroid scintigraphy. The half-life of iodine 123 is of 13.2 hours, which means that the decay takes place over a few days. It takes about 2 weeks for the activity to drop from 37 million becquerels to 1 becquerel, and 23 days for the last nucleus of iodine 123 to disappear.
© IN2P3
The activity of a sample of radioactive matter is defined by the number of disintegrations taking place at its core at any given moment. The activity also represents the number of radiations emitted. One call therefore alpha, beta, gamma activity the numbers of alpha, beta, gamma rays that are emitted and are in proportions of the number of disintegrations. These activities are fundamental features of the radioactive sample and of the type of radiation emitted. They represent its ‘baseline radioactivity’. When the sample contains more than one element, the total activity is the sum of the individual activity values.
In the majority of cases, disintegrations leads to the emission of an alpha or beta ray. The numbers are then equal. If certain decays are accompanied by the emission of gamma rays, a gamma activity is introduced which is in proportion to the emission frequency of these gamma rays.
The activity of a radioelement varies inversely with its lifespan. The longer the half-life of a substance, the lower its activity. An analogy can be made with a soft-burning candle, whose lower flame burns for longer. This is why in uranium 238, which has a half-life of 4.5 billion years, only one nucleus out of 65 million will decay every century.
By contrast, a radioactive nucleus such as oxygen 15 (which is commonly used in medical scans) will disappear within minutes, in the same way that a firework will burn brightly and then vanish. A very small quantity of this radioisotope is all that is needed for significant levels of activity to be observed.

Short lived high activity ; long lived low activity
Activities and half-lives are inversely proportional to each other. Like the explosion of a firework, the activities of short-lived radioisotopes have intense activities but short half-lives.The activity of a long-lived radioactive body can be compared to the light emitted by a glowworm and lasting forever. Uranium 238 which takes billions of years to transform can also be compared to a lake which slowly empties through a pin hole.
©IN2P3
When one measures activity, one counts the number of atomic nuclei that are disintegrating. As the number of atoms present in even the smallest sample of matter is always enormous, the number of disintegrations per second will also be large, even if the proportion of radioactive atoms is low.
The large number of disintegrations allows sensitive detectors to observe infinitesimal doses of radioactive substances and makes extremely accurate measurements. It only takes a millionth of a billionth of a gram of caesium 137, for instance, to carry out efficient sedimentation studies.
One defines the activity of a sample of radioactive material as the number of disintegrations taking place every second. The basic unit of activity is the becquerel (Bq) named after Henri Becquerel, the first man to discover radioactive radiation. A becquerel represents a decay rate of one disintegration per second. For historical reasons, activity values were measured in Curies (Ci) for many years : a Curie is the activity of a standard source of one gram of radium. Such a radium source has 37 billion decays every second, which is a very high value by comparison. As a result, milli- and microcuries were units that were more commonly used. The becquerel is, by contrast, a unit on the same scale as a nucleus: more practical but less realistic. One millionth of a Curie (1 microcurie) is equivalent to 37,000 becquerels (Bq)
The extreme smallness of the becquerel is rarely reminded when by the medias during accidents of radioactivity or nuclear accidents. The absence of this reminder generates unjustified fears.
Other articles on the subject « Radioactive decay law »
Radioactive Half-life
The half-life determines how quickly a radioisotope decays The ‘half-life’ of a radioactive nucle[...]
Radioactive Series
A long Radioactive Lineage A certain number of natural radioactive nuclei are still present on Ea[...]
Radioactive Equilibrium
An equilibrium as old as the Earth Radioactive lineage is the term given to the chain of successi[...]