The very small and the very large …
The atom, and the nucleus in particular, belong to the world of the infinitely small; a world whose minuscule size is almost impossible to grasp. The units and orders of magnitude of our everyday lives no longer have any sense, and so the numbers being used, whether large or small, become extreme. Here are a few helpful points:
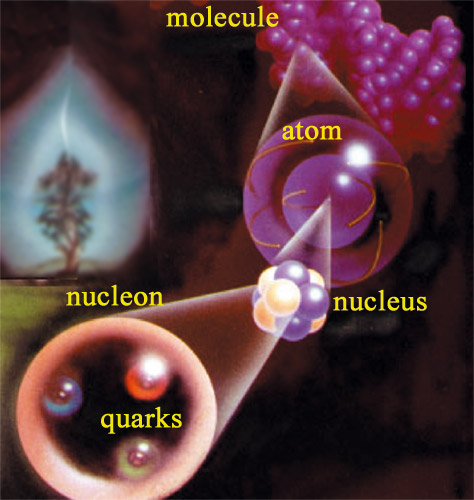
Like Russian dolls …
There are fifteen orders of magnitude separating the scale of our daily lives objects from that of nuclear matter – nuclei, nucleons and quarks – the actors of radioactive phenomena. The living matter in a tree, which can reach 20 or 30 metres into the air, is made of cells containing large molecules formed of minuscule atoms with a radius of several billionths of a metre. As small as they may be, atoms are some 100,000 times larger than the nucleons which agglomerate to form the subatomic nuclei. The Russian doll process has not yet finished: nuclei are in their turn made up of quarks.
© DR
Dimensions: The radii of atoms are of the order of tenths of billionths of a metre. The length of a chain of a billion atoms would barely reach the 20cm mark. A proton is a hundred thousand times smaller, and the length of a similar proton chain would only be 2 microns: two thousandths of a millimeter. Nuclei are somewhat bigger, but not by much.
Speeds: Nothing travels faster than the speed of light in vacuum. It takes light 8 minutes to reach us from the Sun, at a speed of 299,792,458 km/s. The atomic electrons are nowhere near as fast, but their velocity is still of the order of several thousand kilometers per second.
Time scales: It takes light a fraction of a billionth of a billionth of a second to pass through a tiny atom. This minuscule length of time can be viewed as the tick-tock of a pendulum working at the rhythm of the atom: a length of time far smaller than the second. In one second, an energetic electron with a speed of 3,000 km/s – one hundredth of the speed of light – can make as many orbits of the atom as there will be heartbeats on Earth over the coming decade.
Energies: In absolute terms, these tiny particles have infinitesimal energies. But the speeds reached can be very high – out of all proportion to the energies provided by the thermal agitation of the atoms and molecules. An alpha particle with an energy of 4 million electronvolts or MeV, for instance, can be ejected at a speed of 15,000 kilometres per second. Once it slows down and completes its transformation into a helium nucleus, the speed drops to a few kilometers per second and the energy becomes that of most gaseous molecules: a few electronvolts.
When the numbers of nuclei involved become high, the energies released in a nuclear process may turn out to be enormous. Such reactions occur naturally in the stars. In nuclear reactors, fission chain reactions of uranium 235 liberate energies far greater than those released by regular fuels. The energy liberated by the fission of an uranium nucleus is of 200 million electronvolts, 33 million times greater than the energy liberated by the combustion of a carbon atom.
Other articles on the subject « The atomic world »
The Atom
An almost empty space with mass concentrated in a tiny nucleus The atom is often viewed as a mini[...]
The electron
The best known of elementary particles The electron is an elementary particle that plays a fundam[...]
Atomic Energy Levels
A shell structure …. The conquest of space has familiarized us to the concept of a satellit[...]
Photons
The elementary components of light and electromagnetic waves Light is composed of infinitesimal i[...]
Avogadro’s Number
Trillions of billions of very small atoms …. The microscopic size of atoms comes with their[...]
E=MC²
The Einstein formula, a relation between mass and energy The energy released by a chemical reacti[...]