A long Radioactive Lineage
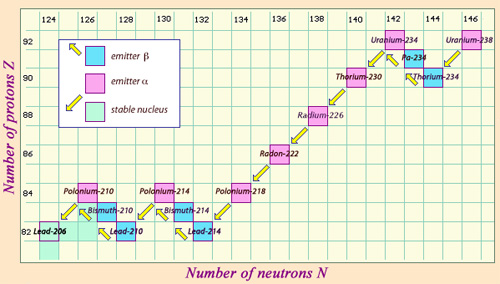
From Uranium 238 to Lead 206
This diagram maps the journey on the decay chain of an uranium 238 nucleus. Alpha decays cause the number of protons and neutrons to diminish by 2, whereas beta-negative decay diminishes the number of neutrons by 1 and increases the number of protons by 1. The instability caused by alpha decays is sometimes corrected by beta decays. Finally a stable nucleus of lead 206, with its 82 protons and 124 neutrons, ends the journey.
© IN2P3
A certain number of natural radioactive nuclei are still present on Earth, even though their half-lives are particularly short when compared to our planet’s age. These radioisotopes are the descendants of three heavy nuclei with very long half-lives : uranium 235 (with a half-life of 0.7 billion years), uranium 238 (which lives for 4.47 billion years) and thorium 232 (with a half-life of 14.0 billion years).
These three ‘patriarchs’, to extend the metaphor of the radioactive family, were all present in the proto-star: the cloud that eventually condensed to form our Sun, the Earth, and the planets. Each of the three is the ancestor of a distinct family of natural radioactive elements, perhaps the most important of which is that of uranium 238.
A nucleus of uranium 238 decays by alpha emission to form a daughter nucleus, thorium 234. This thorium in turn transforms into protactinium 234, and then undergoes beta-negative decay to produce uranium 234. This last isotope changes slowly (with a half-life of 245,000 years) into thorium 230, yet another unstable nucleus.
Any such decay chain is only stopped by the formation of a stable nucleus. This occurs at the fourteenth generation of the uranium 238 family, when lead 206 is finally produced. The two other families, those formed from uranium 235 and thorium 232, end respectively with the creation of lead 207 and lead 208, two other stable isotopes of lead.
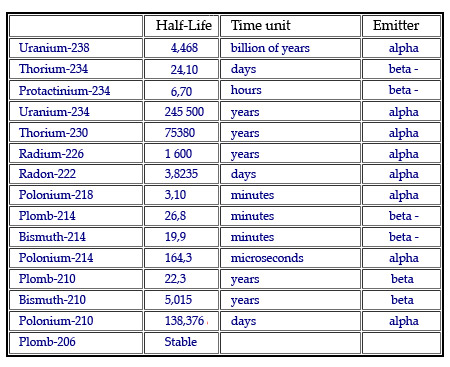
The 14 generations of the uranium 238 lineage
Simplified radioactive lineage of uranium 238. The consecutive decays with drastically different half-lives change the basic structure of the nucleus and hence of the atom. The total number of nucleons goes down by 4 when the nucleus emits an alpha particle and does not change when beta negative emission takes place.
© IN2P3
The half-lives are all extremely variable, and it is difficult to represent a range of timescales going from individual seconds to billions of years. In this sense the lineage of a nucleus resembles the flow of water over mountains and plains: torrential at one point and lazily winding at another.
As is normal for the heaviest nuclei, alpha decay is particularly common in all three decay chains. With each emission causing a loss of two protons and two neutrons, however, the neutron : proton ratio increases as we move down the family tree. As a result, beta decay is needed to even up the balance. In the Uranium-238 lineage for example, the first alpha decay is followed by two successive beta decays transforming a thorium 234 nucleus into uranium 234.
Alpha decay causes a loss of four nucleons whereas beta decay has no effect on the number of nucleons present. This is why descendant nuclei always have a multiple of four nucleons less than their ancestors: as can be seen with uranium 238.
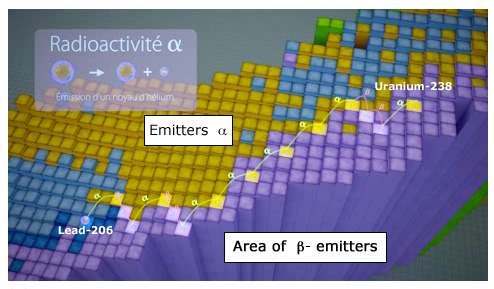
Path of uranium-238 filiation
Path of an uranium-238 nucleus descendants on the nuclides map showing the stability valley. Along this route, alpha emissions decrease the nucleus size, beta emission correct neutrons in excess. It will take billions of years for an uranium-238 nucleus to reach the terminus, a stable lead-206 nucleus.
© CEA-IRFU
The members of the uranium 238 family, therefore, have 4n+2 nucleons, whereas uranium 235 family has 4n+3 and thorium family 232 has 4n nucleons. In principle, the fourth (4n+1) family should exist, but its ancestor, neptunium 237, has a relatively ‘short’ half-life of 2.14 million years. As a result, this family has had ample time to disappear since the nucleosynthesis of neptunium 237 in the cores of the stars which preceded our Sun.
Other articles on the subject « Radioactive decay law »
Radioactive Half-life
The half-life determines how quickly a radioisotope decays The ‘half-life’ of a radioactive nucle[...]
Radioactive Activity
The decay rate and the rate of radiation emitted The activity of a sample of radioactive matter i[...]
Radioactive Equilibrium
An equilibrium as old as the Earth Radioactive lineage is the term given to the chain of successi[...]