A pure gamma emitter widely used in nuclear medicine
Of all the atoms below uranium in Mendeleyev’s periodic table, there are only two that are so unstable as not to occur in nature. One is promethium, with a nucleus containing 61 protons, and the other has an atomic number of 43: technetium.
An exceptional nucleus
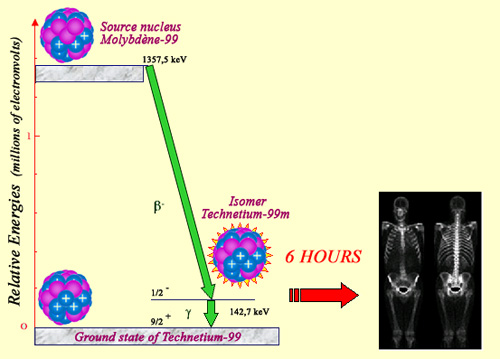
Technetium 99m, widely used in nuclear medicine, is a long-lived intermediary step in the life of a technetium nucleus obtained through the decay of molybdenum 99. According to the flowchart, the emission of a beta electron leads to an excited nucleus, which returns to its ground state by emitting a gamma photon. This excited state persists for a few hours, which gives hospitals the time to isolate it, inject it into a patient as a radioactive marker to map the body, for example through a bone scintigraphy.
© IN2P3
Technetium, which is chemically similar to potassium, is something of a freak. The half-lives of its three isotopes range between 215,000 and 2.6 million years; long, but extremely short when compared to the Earth’s age of 4.5 billion years. Technetium has therefore had plenty of time to completely disappear from our planet.
For the last fifty or so years, technetium 99 has been artificially created as a fission product in nuclear reactors. This technetium 99 , with a half-life of 215,000 years, is the most abundant member of the family of long-lived fission products. It is relatively mobile. Considering the disposal of nuclear waste, this relative abindance and mobility makes it the main contributor to the very long-term potential radiotoxicity of the fission products. Along with iodine 129 and caesium 135, it is one of the nuclei whose elimination through transmutation is the object of a program of research and developments that may reduce the dangers of long lived fission products in the coming millennia.
‘Technetium 99m‘ is a long-lived isomer of technetium 99, widely used in nuclear medicine. The term ‘isomer’ refers to those nuclei that are able to survive in excited states for abnormally long periods of time. Excited nuclei normally return to their ground states after a fraction of a second, though in rare cases this transition can be inhibited and greatly slowed down. This is the case with technetium 99m, which exists for several hours before returning to the normal state of technetium.
This excited state has a name (technetium 99m) that distinguishes it from ordinary technetium 99, as the 6-hour half-life of technetium 99m gives researchers enough time to put these nuclei to use. In order to leave the excited state, technetium-99m nuclei emits 140 keV characteristic gamma rays without accompanying beta rays. This property makes them highly desirable in medicine. Gamma rays are absorbed far from the examined organ, minimizing the danger posed to living matter.
The technetium-99 precursor is molybdenum-99, a radioactive nuclei generally produced in reactors. The 66 hours molybdenum radioactive half-life give enough time to transport it to hospitals and to extract chemically technetium 99m. The radioisotople placed in a radiopharmaceutical serum is then injected into the patient, which allows gamma camera scans providing accurate pictures of the patient’s body. Technetium 99m is the most commonly used radioisotope in the field of nuclear imaging being involved in 80% of scintigraphies.
Other articles on the subject « Main Radioactive Nuclei »
Uranium 238 and 235
A radioactive and strategic element The uranium atom is the heaviest atom present in the natural [...]
Plutonium 239
Plutonium 239: an artificial fissile nucleus, highly sought-after and feared Plutonium, the ninet[...]
Plutonium Properties
A transuranic element with long-lived radiotoxic isotopes Plutonium is a very dense metal, radioa[...]
Radium
The radioactive nucleus that made History Radium is an extremely rare element that was first disc[...]
Carbon-14
A by-product of cosmic rays The nucleus of carbon 14 contains 6 protons and 8 neutrons, instead o[...]
Potassium-40
A curiosity of Nature and a very long lived beta emitter Potassium 40 is a radioisotope found in [...]
Iodine 131
Radioactive iodine : A dangerous and short lived fission product Iodine 131 is a radioisotope wit[...]
Tritium
A radioactive isotope of hydrogen Tritium is a beta-emitting radioactive isotope of hydrogen. Its[...]
Caesium 137
A legacy of atmospheric nuclear bomb tests and accidents Caesium 137 is a radioactive element wit[...]
Strontium-90
A fission product with properties close to calcium Strontium-90 is with cesium-137 a major radioa[...]