The charged constituent of the nucleus
Structure of the proton
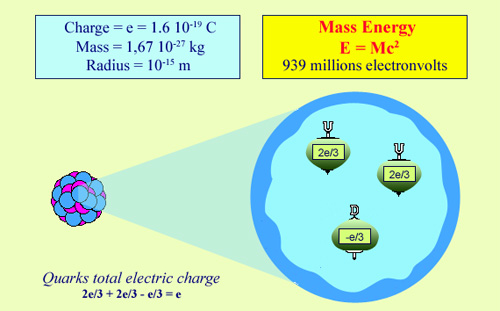
Nuclei are made up of two similar components called nucleons: the proton and the neutron. The positive electrical charge of the proton is equal and opposite to the negative charge atomic electrons. This charge, called ‘e’, is an ‘elementary’ electric charge. The proton is a very small particle with a radius of under a millionth of a billionth of a meter. Despite its size, the proton is a complex particle made up of even smaller particles: quarks. For most phenomena linked to radioactivity, however, this internal structure can be ignored.
© IN2P3
The nucleus of the hydrogen atom consists of one solitary particle with a positive electric charge: a particle known as a proton. The value of this electric charge e is 0.16 billionth billionth of a Coulomb, the standard unit for measuring electric charge. All known particles, with the exception of quarks, have charges which are whole number multiples (either positive or negative) of this electric charge, which is therefore referred to as the ‘elementary’ charge.
The charge on the proton has exactly the same value as that of the electron, but is positive rather than negative. It is the balance between these equal yet opposite charges of the protons and electrons that assures the electric neutrality of the atom. The number of protons in an atomic nucleus is conventionally called ‘Z’; giving the nucleus a total charge of Ze (a positive multiple of the proton’s charge). In an atom, the number of electrons surrounding the nucleus is also Z.
The proton, however, is not a fundamental particle. If we represent a proton by a tiny sphere, then the value of the radius is of the order of 1 fermi – a thousandth of a billionth of a millimeter. Its mass is 1836 times that of the electron but is still very small: 1.672 thousandths of a billionth of a billionth of a kilogram.
Science has shown since the 1970’s that protons, like neutrons, are built up of smaller particles known as ‘quarks’. These quarks are held together by intense attractive forces, and are, like the electron, fundamental particles. The ‘strong force’ (sometimes referred to as the ‘colour force’) that holds these quarks together is determined by the ‘strong charge’ or ‘colour charge’ they have – a property that has nothing to do with the electric charge or ordinary colour. Contrarily to quarks, protons end neutrons are said to be ‘colourless’ : their total strong charge is null.
A parallel between electric charges and the “strong” charges
The balance between electric charges is what allows for the formation of atoms; assemblies of nuclei and electrons whose positive and negative charges cancel each other out. Then, atoms join together to form electrically neutral molecules by sharing electrons, and the electrical charge they carry.
By the same token, the ‘strong’ charges bring quarks together to form protons and neutrons. Inside these nucleons, the strong charges balance each other in order to produce a net strong charge of zero. Like atoms combining to form molecules, these nucleons group together in nuclei, the equivalent of molecules for the strong forces.
The strong nuclear force that keeps a nucleus together can be compared to the complex forces that bind atoms to form molecules. Just as electrons join together to form an atom, nucleons agglomerate into nuclei when they come into contact. This nuclear glue is short range and makes the nuclei very compact, preventing the strong force from being felt outside.
Other articles on the subject « The atomic nucleus »
The Neutron
The neutral partner of the proton The neutron is, along with the proton, one of the two constitue[...]
Isotopes
Nuclear variants of a given atom … Free of all electric charge and present only in the nucleus, n[...]
Nucleus Energy Levels
Analogies with the atomic energy levels … At first glance, nuclei seem to be very different[...]
Quarks and leptons
The fundamental constituents of matter On the most elementary scale, the world around us is made [...]
Quarks and Gluons
How quarks interact within nuclei … To describe radioactivity and nuclear reactions such as[...]
Matter and Antimatter
Nature loves symmetry. However, one can not dream of a matter less symmetrical than the matter wh[...]