A by-product of cosmic rays
The nucleus of carbon 14 contains 6 protons and 8 neutrons, instead of the 6 and 6 found in ordinary carbon 12. The imbalance makes carbon 14 a radioisotope with a half-life of 5,700 years, and an emitter of beta particles. This radioactive isotope of carbon is called radiocarbon.
The carbon 14 found in nature is constantly being regenerated by cosmic rays hitting the atmosphere. The rate at which the regeneration takes place has gone virtually unchanged for centuries; a feature which depends on the flux of particles bombarding the earth, and the strength of the magnetic field capable of diverting them. This magnetic shield, and consequently the particle flux, has slowly changed over time, and the quantity of carbon 14 formed on Earth changes with it.
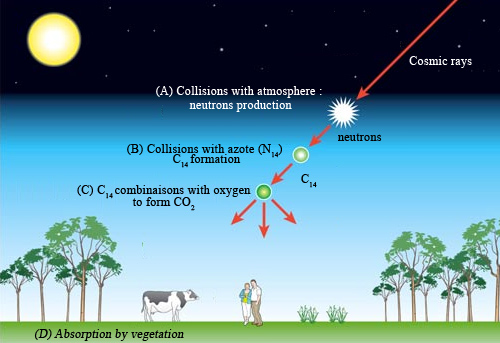
Steps of Carbon-14 Formation
Carbon-14 is continuously generated in the atmosphere by cosmic radiation. Neutrons are ejected from nuclei of the upper atmosphere in collisions with cosmic rays (A). Captured by nitrogen nuclei (N-14), neutrons transform these nuclei into carbon-14 (B). The carbon-14 atoms combine with the oxygen in the air to form carbon dioxide (C). Finally, CO2 molecules with radiocarbon, absorbed by vegetation, enter natural environment (D).
© IN2P3
Incoming cosmic rays create atoms of carbon 14 by colliding with nuclei in the upper atmosphere, liberating neutrons. These neutrons in turn interact with nuclei of nitrogen in the air, replacing one of the 7 protons nitrogen contains with an extra neutron. The resulting atom, now containing 6 protons and 8 neutrons, is one of carbon 14
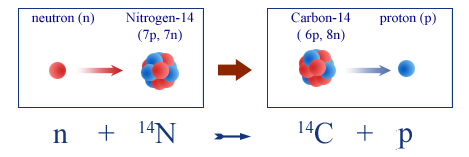
Formation of carbon-14 from atmospheric nitrogen.
© IN2P3
Carbon gases formed with carbon 14 are chemically indistinguishable from gases with the ordinary carbon, carbon 12. The radioactive atom is absorbed by plants and living matter in the same way as its non-radioactive isotope ; in every thousand billion (ten to the power of twelve) atoms of carbon 12, there will be on average one atom of carbon 14.
This tiny ratio exists in all molecules involving carbon atoms, including all living matter. This is why carbon 14, along with potassium 40, accounts for almost all the natural radioactivity of our body.
When a living organism dies, the radioactive carbon is no longer absorbed, and the ratio of carbon 14 present begins to decrease. The amount still present in a sample of what was once a living creature can thus be used to determine its age.
Carbon 14 can also be used as a radioactive marker.
Other articles on the subject « Main Radioactive Nuclei »
Uranium 238 and 235
A radioactive and strategic element The uranium atom is the heaviest atom present in the natural [...]
Plutonium 239
Plutonium 239: an artificial fissile nucleus, highly sought-after and feared Plutonium, the ninet[...]
Plutonium Properties
A transuranic element with long-lived radiotoxic isotopes Plutonium is a very dense metal, radioa[...]
Radium
The radioactive nucleus that made History Radium is an extremely rare element that was first disc[...]
Potassium-40
A curiosity of Nature and a very long lived beta emitter Potassium 40 is a radioisotope found in [...]
Iodine 131
Radioactive iodine : A dangerous and short lived fission product Iodine 131 is a radioisotope wit[...]
Tritium
A radioactive isotope of hydrogen Tritium is a beta-emitting radioactive isotope of hydrogen. Its[...]
Caesium 137
A legacy of atmospheric nuclear bomb tests and accidents Caesium 137 is a radioactive element wit[...]
Strontium-90
A fission product with properties close to calcium Strontium-90 is with cesium-137 a major radioa[...]
Technetium 99
A pure gamma emitter widely used in nuclear medicine Of all the atoms below uranium in Mendeleyev[...]