Three nuclear forces and their hierarchy
Three types of force act alongside each other inside a nucleus. The dominant one is the nuclear or ‘strong’ interaction which ensures the cohesion of the nucleus by pulling the various nucleons together, a force which is also responsible for the production of alpha radiation. Secondly, the electromagnetic repulsion among the charged protons, but is considerably less powerful than the strong force. The third of these nuclear interactions is the ‘weak’ force ; neither attractive nor repulsive, it acts inside the individual nucleons and can occasionally lead to a neutron transformation into a proton (or vice versa), accompanied by a release of beta radiation. The interplay between these three forces dictates how stable or unstable a nucleus is.
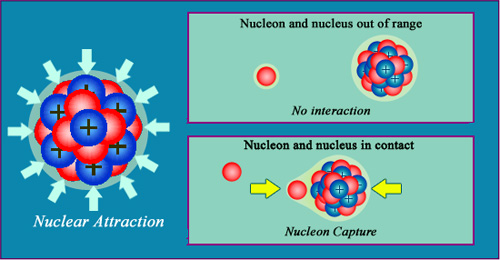
A very strong short range attraction
The cohesion of the nuclear structure is mainly due to the attractive strong force, which is capable of overcoming the electrical repulsion the protons exert on each other. This ‘strong force’ is only effective over short distances, as a nucleon passing very close to a nucleus will not feel its influence. The force only starts to apply when direct contact is made – to symbolise this ‘nuclear glue’, the nucleons and nuclei above have been drawn with a layer of glue surrounding them.
© IN2P3
All nuclei are practically incompressible like the molecules of a liquid. This is caused by the direct contact that exists between the constituent neutrons and protons.
The nucleons are all held together by a contact force, called nuclear or strong, which is the dominant force inside the nucleus. Despite being remarkably powerful, this nuclear glue only acts over the shortest of distances. This explains why these forces went undetected for decades even after the discovery of radioactivity. These distances are so short, in fact, that neutrons can travel in the immediate vicinity of a nucleus without being affected by the force and eventually absorbed into the nucleus.
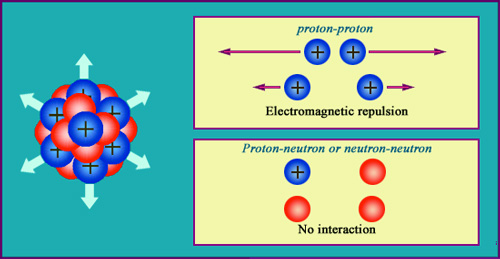
The electrical repulsion of protons
Two electrical charges of the same sign repel each other. This repulsion increases with the inverse of the square of the distance, as dictated by Coulomb’s law. The protons (in blue) are affected by this repulsion inside the nucleus, as opposed to the red neutrons, whose absence of charge makes them immune. Without the strong nuclear forces capable of overcoming these repulsions, the nucleus would explode. The strong force needs to be very intense to hold the protons together in such a small volume.
© IN2P3
The electromagnetic repulsion takes place within the nucleus between like electric charges. These charges are carried by the protons, whose close proximity to each other intensifies this repulsive force. The strong nuclear attraction must be immensely powerful to overcome the repulsion taking place in a sphere whose radius is only of a few millionths of a billionth of a metre.
The third nuclear force is a discreet one that nevertheless plays a fundamental role in the universe. Without the ‘weak force’, our Sun would stop shining due to the inability of hydrogen nuclei to fuse to form deuterium, the Sun’s main energy-producing reaction.

The tritium “weak decay”
The example of tritium, the simplest of radioactive nuclei, shows how nature occasionally uses the ‘weak forces’ to change the proportions of protons and neutrons. One may suppose that tritium, given the fact that it contains one proton and two neutrons, could eject one of its two neutrons to reach stability. Such an expulsion, however, would require too much energy to occur, and so one of the neutrons is transformed into a proton, accompanied by the release of a beta electron and an antineutrino. This process releases enough energy to occur and make tritium radioactive; an instability that is caused by the weak force.
© IN2P3
Without the weak force, there would be a great deal more than 287 ‘natural’ nuclei in the world. In the absence of beta radioactivity, the only way for a nucleus to maintain the correct proton/neutron balance would be the expulsion of a proton or neutron. This requires energy and do not occur naturally. Without the weak force which allows neutrons to transform into protons (and vice versa) at a cheap price, several thousands of recorded unstable and radioactive nuclei would be stable.
Other articles on the subject « Radioactive nuclei »
Map of Nuclei
Map of stable and unstable nuclei The progress made in our understanding of the subatomic world o[...]
Stability Valley
Beta decay : nuclei getting slimmer to achieve stability The nucleus mass is related to its inter[...]
Mecanisms of Radioactivity
The way radioactivity works and its origins The impressive range of half-lives that exist, extend[...]
α decay : tunnel effect
Particle and wave: an effect of quantum mechanics The great age of uranium and thorium nuclei tha[...]
Alphas with gammas
Gamma rarely accompanies alpha decays It is surprising that one can hold with hands a sample of p[...]
β decay : weak forces
The forces which allow a nucleus to emit beta electrons Beta decay (β) and electronic capture cha[...]
Weak Forces
A special and fascinating fundamental interaction A third force is at work in the nucleus next to[...]
The Nucleosynthesis
Primordial and stellar nucleosynthesis The Universe hasn’t always existed, It was born a li[...]
Nucleosynthesis (continued)
Mechanisms of atomic nuclei formation Most of the nuclei of atoms that make up our daily life wer[...]