Radioactive iodine : A dangerous and short lived fission product
Iodine 131 is a radioisotope with a very short period (half-life) of 8.02 days, making it highly radioactive. Frequently used in small doses in thyroid cancers therapies, it is also one of the most feared fission products when accidentally released into the environment.
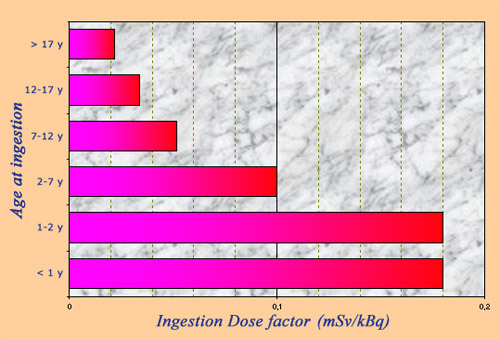
Radiotoxicity of iodine 131
The radioactive toxicity of iodine 131 is measured by an ‘ingestion dose conversion factor’ which allows to calculate the effective dose resulting from the ingestion of a given activity of a radioelement. Ingested Iodine-131 is dangerous because it primarily affects the thyroid gland that plays a fundamental role in childhood development. Radioactive iodine toxicity varies greatly with age, with toddlers, young children and adolescents being far more sensitive than adults.
© IN2P3
In medicine, iodine 131 is primarily used to study the functionning of the thyroid though it can also be employed in the treatment of hyperthyroidism as well as thyroid cancer. The first production of iodine 131 in France took place in 1949 at the Fort de Chatillon, the site of the first Zoe atomic reactor, before its was transfer to the nuclear research centre at Saclay. The isotope had been used since 1942 in the treatment of thyroid cancer.
Though used in low doses for medical examinations, iodine 131 is an ideal tracer for use in humans. Only a few radioactive atoms need to be inserted into the bloodstream for the iodine path to be accurately monitored. The atoms integrate into molecules that eventually transform into thyroid hormones; this is particularly interesting, given that iodine attaches itself exclusively to the thyroid gland. Gamma ray scintigraphy scans can thus monitor the thyroid activity and flag up the appearance of any anomalies. In recent years, iodine 131 has been abandoned in favour of another isotope, iodine 132 – a gamma emitter with a half-life of only 13.2 hours.
Stronger doses of iodine 131 are also used in radioactive therapies aimed at dealing with thyroid cancers. Iodine is inserted into the bloodstream in the same manner, and the short trajectory of the emitted beta particles guarantees that the radiation only affects a comparatively small part of the body.
Iodine 131 is also a feared fission product, posing as it does the principal risk for short-term contamination in the event of accidental waste release. From a chemical point of view, iodine is a halogen (similar in structure to chlorine and fluorine) and its high volatility means that it easily transforms into a purple vapour.

March 2011 : spinachs contaminated by iodine-131
Spinach grown in north-eastern Japan were contaminated by radioactivity of the Fukushima accident in March 2011. Radioactive dust have deposited iodine-131 atoms on spinach broad leaves, leading to its selling interdiction. Iodine-131 is the most feared radioactive release after a nuclear accident because of its fixation by the thyroid. But – counterpart of its high activity – the amount of iodine-131 is divided by 2 every 8 days, by 2500 every 3 months. After a year no iodine traces remain in food.
© Eugene Hoshiko / AP
Volatile and highly mobile in the environment as volatile, radioactive iodine isotopes follow the usual transfer processes to the food chain : dispersion, deposits, uptake by plant leaves, root absorption, ingestion by animals and humans. Ingested by animals during lactation, iodine deposited on the grass finds its way in milk a few hours after ingestion, the maximum appearing after three days.
After a nuclear or radioactivity accident, the iodine-131 should be monitored in the food chain forseveral weeks, until it disappears radionuclide, specially in milk and vegetables, especially and large leaves vegetables like spinach and lettuce. Water should also be monitored.
In any case, the high radioactivity of iodine 131 is somewhat offset by its high decay rate, with the level of activity dropping by a factor of 1000 every eighty days. There are also procedures to protect ourselves before it decays.
Other radioactive isotopes of iodine have very short lifetimes such as iodine-132 and iodine-133 whose periods are 20.8 and 2.3 hours. These isotopes deliver almost their radiatioactivity in the early days after a reactor is shutdown.
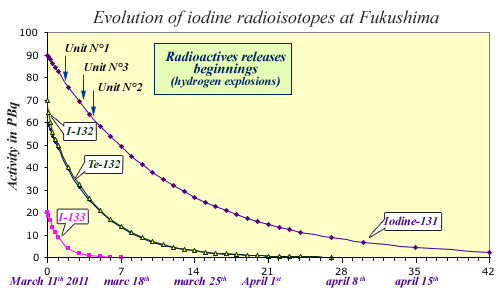
Radioactive decays of shortlived iodine radioisotopes
Besides iodine-131, two other radioactive isotopes are to be considered after a nuclear accident : iodine-133 and 132 (20.8 hours and 2.3 hours periods). Iodine-132 comes from the decay of tellurium-132 (3.2 days period). Using data published after the Fukushima accident, the figure displays the activity of iodine radioisotopes during the first seven weeks after the accident. The releases in the environment occured 1 to 3 days after the tsunami and reactors shut-downs. Iodine-133 had already practically vanished at the time of the first releases. Iodine-132 that closely follows tellurium decay disappears within three weeks. At the end of the seven weeks, only remains Iodine-131 that has decreased 38 times.
© IN2P3
Iodine 129, another isotope of iodine, is one of the long-lived fission products that have to be taken into account when dealing with radioactive waste. The half-life of iodine 129 is of 15.7 million years.
Other articles on the subject « Main Radioactive Nuclei »
Uranium 238 and 235
A radioactive and strategic element The uranium atom is the heaviest atom present in the natural [...]
Plutonium 239
Plutonium 239: an artificial fissile nucleus, highly sought-after and feared Plutonium, the ninet[...]
Plutonium Properties
A transuranic element with long-lived radiotoxic isotopes Plutonium is a very dense metal, radioa[...]
Radium
The radioactive nucleus that made History Radium is an extremely rare element that was first disc[...]
Carbon-14
A by-product of cosmic rays The nucleus of carbon 14 contains 6 protons and 8 neutrons, instead o[...]
Potassium-40
A curiosity of Nature and a very long lived beta emitter Potassium 40 is a radioisotope found in [...]
Tritium
A radioactive isotope of hydrogen Tritium is a beta-emitting radioactive isotope of hydrogen. Its[...]
Caesium 137
A legacy of atmospheric nuclear bomb tests and accidents Caesium 137 is a radioactive element wit[...]
Strontium-90
A fission product with properties close to calcium Strontium-90 is with cesium-137 a major radioa[...]
Technetium 99
A pure gamma emitter widely used in nuclear medicine Of all the atoms below uranium in Mendeleyev[...]