An almost empty space with mass concentrated in a tiny nucleus
The atom is often viewed as a miniature solar system; a representation that goes back to the first modern atomic model proposed in 1913 by the Danish physicist Niels Bohr. Though a pleasing analogy it is a somewhat misleading one, as the atom is in fact far more complicated. The constituents of the atom, the nuclei and electrons, all obey the laws of quantum mechanics.
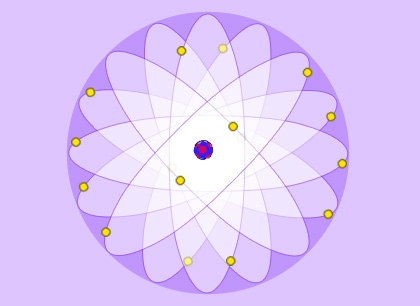
The familiar image of the atom
Atoms are generally represented as miniature solar systems. This classical image with orbits, describes a system where practically all the matter is concentrated in a tiny volume at the centre. The rest of the atom consists of empty space where electrons, particles several thousands of times lighter than the nucleus, travel around it. The nucleus is a hundred thousand times smaller than the atom and the electrons, rather than going in precisely-defined orbits, share up the empty space between them.
© IN2P3
Atoms consist of a group of electrons rotating around a nucleus that is some 100,000 times smaller.
Electrons are microscopic particles with an electrical charge of –e – the smallest electrical charge it is possible to have. All other values of electrical charge are whole number multiples of this charge e, which is known as the elementary charge.
The number of electrons present in an atom, which can vary from one to a hundred, defines the atomic number of the neutral atom; that is to say, one that has not captured or lost any electrons (as we will see later, this number is equal to the number of protons, Z, in the nucleus). The simplest atom is that of hydrogen, which has one electron orbiting a solitary proton. Oxygen for instance has eight electrons arranged in layers or ‘shells’, with the outermost shells defining the chemical properties of the atom in question.
The electrical charge of the body of electrons is exactly equalled by the positive charge present in the nucleus. The protons, which have an electrical charge equal and opposite to that of the electron, need to be present in the same quantity as electrons for the atom to be neutral.
Inside the nucleus, alongside the protons, we find an approximately equal number of neutrons; particles similar in size to the protons but with no electrical charge.
The collective term for a constituent of the nucleus (whether a proton or a neutron) is a nucleon. Protons and neutrons have pratically the same mass, which is about two thousand times greater than that of an electron. It is the nucleus, therefore, which is the principal contributor to the mass of the atom. The vast majority of the atom’s mass is confined in the infinitesimal volume occupied by the nucleus, a space that has a density of about 230,000 tons per cubic millimeter.
A, the number of nucleons an atom contains, is known as the mass number. It turns out that the ratio between the masses of two atoms is practically equal to the ratio between their mass numbers. As a result, an atom of oxygen (with a value of A=16) will be almost exactly 16 times heavier than an atom of hydrogen (A=1).
Other articles on the subject « The atomic world »
The electron
The best known of elementary particles The electron is an elementary particle that plays a fundam[...]
Atomic Energy Levels
A shell structure …. The conquest of space has familiarized us to the concept of a satellit[...]
Photons
The elementary components of light and electromagnetic waves Light is composed of infinitesimal i[...]
Orders of Magnitude
The very small and the very large … The atom, and the nucleus in particular, belong to the [...]
Avogadro’s Number
Trillions of billions of very small atoms …. The microscopic size of atoms comes with their[...]
E=MC²
The Einstein formula, a relation between mass and energy The energy released by a chemical reacti[...]