Plutonium-239 but also 238, 240, 241, 242 …
Plutonium isotopes are produced by neutron capture in a uranium-based nuclear fuel. When this element is not extracted from the nuclear spent fuel and recycled as fissile material, its radioactive toxicity becomes predominant after a few decades although it represents only 1% in mass of the spent fuel.
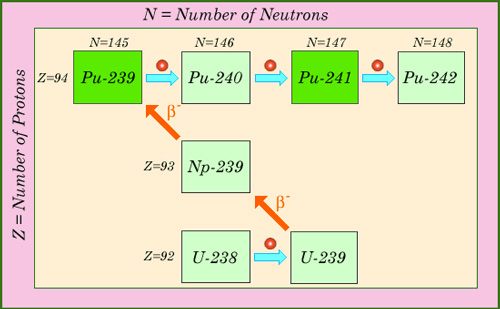
Successive neutron captures
Multiple captures of neutrons are responsible for the formation of plutonium isotopes and actinides in reactors. This map of nuclei shows how a succession of neutron captures and beta decays generates the isotopes of plutonium. In the case of plutonium-239 and plutonium-241, considered fissile, the capture of slow neutrons triggers often a fission. Fast neutrons are able to split other isotopes of plutonium.
© IN2P3
Because of the prominent role played by plutonium-239, one often forget that there are other plutonium isotopes. In the case of conventional pressurized water reactor burning uranium fuel, plutonium 239 corresponds to 58% of plutonium found in the spent fuel. A second isotope, plutonium-241 is also fissile, bringing to 70% the proportion of fissile plutonium isotopes.
Other plutonium isotopes present in spent fuels are plutonium 238, 240 and 242. Plutonium-238 is produced from neutron captures in uranium-235 not followed by fission.
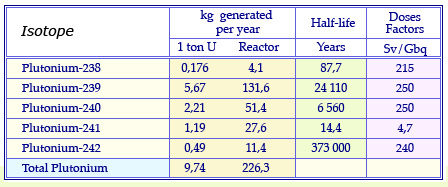
Plutonium isotopes from reactors
Masses of plutonium isotopes produced annually in the fuel of a conventional pressurized water reactor having undergone standard “burn-up” of 33 Gigawatt-day after 3 years spent in the reactor. These quantities are given for 1 uranium ton and the annual consumption of 23 uranium tons of a reactor. The table quotes also the half-life of these isotopes and their radiotoxicity dose factor, an indicator of their potential toxicity.
© Source CEA
Plutonium radioactivity comes mainly from two relatively short lived isotopes: plutonium 241 (14.4-years half-life or period) and plutonium 238 (88-years period). Plutonium-241 is produced from uranium-235, as a result of neutron captures not followed by fission. Plutonium-238 is used as an energy source in remote spatial missions. Its radioactive period close to a century ensures a long use.
Although present only at a 12% level, plutonium-241 contributes to virtually 100% of the initial activity of plutonium. However, this beta emitter (it turns into americium-241) is less radiotoxic than the isotopes 238, 239, 240 and 242, which are alpha emitters.
The composition and the radioactivity of plutonium vary with its age. Soon after the removal of the spent fuel from the reactor core, plutonium 238 is the main contributor to its radiotoxicity because of its lifetime of about one hundred years. Over long time scales, the longer-lived isotopes 239, 240 and 242 will take the lead , but owing to these relatively short-lived isotopes, the plutonium activity and toxicity wille have declined.
Plutonium is responsible for 97.5% of the activity and 66% of the initial potential toxicity of actinides in the fuel taken out of the reactors. If it is not recycled and left in the fuel, plutonium will become the main source of toxicity when fission products have virtually disappeared. However, in the case of a reprocessing where it is removed from the fuel, the residual radioactive toxicity of the waste (due to minor actinides) is divided by 7.
The isotope composition obviously depends on the fuel used and its degree of irradiation – the burn-up – when it is removed from the reactor. In particular, fuel including plutonium initially – such as MOX – contains more plutoniun at the end.
Other articles on the subject « Nuclear Fission »
200 millions electronvolts !
A huge amount of energy at the atomic scale The fission of a uranium or plutonium nucleus liberat[...]
Chain Reaction
From one single fission to fissions on a massive scale Nuclear fission emits a lot of energy on t[...]
Fissile nuclei
Uranium 235 and 233, plutonium 239 The few fissile nuclei found in nature belong to heavy atoms, [...]
Fission fragment
Chronicle of the life of a fission fragment … The fission of a nucleus into two fragments i[...]
Fission products
The ashes of nuclear fission Fission products are the remains of a heavy uranium or plutonium nuc[...]
Plutonium-239 formation
Transforming a fertile nucleus into a fissile nucleus Uranium-238 accounts for more than 95% of t[...]
Short-lived Fission Products
Most fission products vanish rather rapidly The vast majority of the radioactive fission products[...]
Long-lived Fission Products
A handful of tough but little radioactive isotopes … The more a nucleus is long-lived, the [...]
Actinides
A class of very heavy radioactive nuclei Alongside the fission products found in the core of nucl[...]
Minor Actinides
Neptunium, americium and curium Minor actinides constitute a very small minority of high activity[...]