From one single fission to fissions on a massive scale
Nuclear fission emits a lot of energy on the scale of tiny nuclear nuclei. If fission was limited to a few nuclei of uranium-235 or plutonium_239 the energy released would still remain very small at our scale. On the other hand, if the reaction involves a very large number of nuclei, it is on our scale that this large release of energy will manifest itself.
It is the chain reaction process that is used in reactors and nuclear weapons to generate a large number of fissions. In a reactor, the propagation of fissions takes place in a controlled manner, in a nuclear weapon in an uncontrolled, explosive manner.
The products of nuclear fission are usually accompanied by two to three secondary, free neutrons which can be absorbed later by other fissile nuclei. These absorptions in turn lead to further fissions, then to the production of other neutrons etc …, a process commonly called chain reaction.
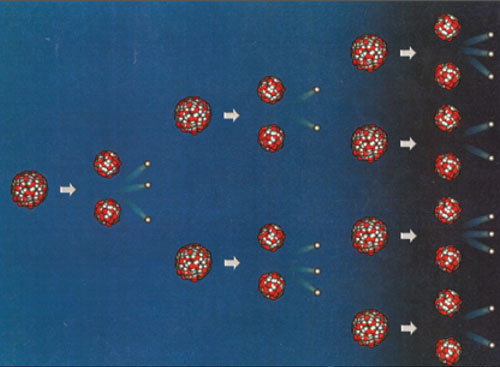
Principle of a chain reaction
Following the absorption of a neutron, a fissile nucleus of uranium 235 or plutonium 239 splits into two unequal fragments and release several secondary neutrons. In the diagram above, two of these ‘secondary’ neutrons lead to fission reactions which, in turn, release neutrons capable of causing more fission reactions. When the proportion of fissile nuclei is very high this chain reaction can become explosive, a property used to full effect inside atomic bombs. In the core of a reactor containing less than 4% fissile nuclei, the fuel is designed in such a way that each fission produces exactly one ‘torch bearer” neutron, leading to a continuous but stable chain reaction.
© GSI
Plutonium-239 is the fissile nucleus that produces the most secondary neutrons : 2.91 in average compared to 2,47 for uranium-235 and 2.55 for uranium-233. The number of neutrons available for new fission depends on the abundance of fissile nuclei in the nuclear fuel and on the amount of losses due to neutrons leaving the core of the reactor or being trapped by other nuclei without fission.
When the number of the useful neutrons, available for carrying the torch of the fission reaction, is greater than one, the chain reaction takes on a dangerously explosive character. Instead of involving a few individual nuclei, the chain reaction propagates through a significant fraction of the fuel. The fission energies being several million times greater than any chemical reaction, the explosion releases enormous energy significant on the human scale.
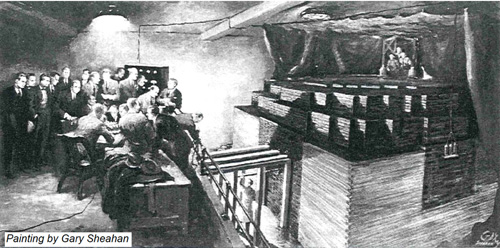
Chicago 1942 : first chain reaction
It was on December 2, 1942, during World War II, that a team of American physicists led by Enrico Fermi achieves the first nuclear chain reaction. The event took place in Chicago, under the stands of a stadium, within the first atomic pile ancestor of our reactors in the framework of the Manhattan Project.
© DR
Nuclear reactors are to function at a safe level, in a continuous way during weeks, months and years. It is important to have absolute control over the neutron capture process. Reactors powered by natural uranium are the more difficult to operate, owing to the very low concentration (0.7%) of the fissile uranium 235 in natural uranium. Most modern reactors burn nuclear fuels that are enriched from 0.7% up to a 3.5-4%. level, using the techniques of Isotopic separation. This allows enriched uranium to be used in pressurised or boiling water reactors, by far the most common type of reactor functioning today.
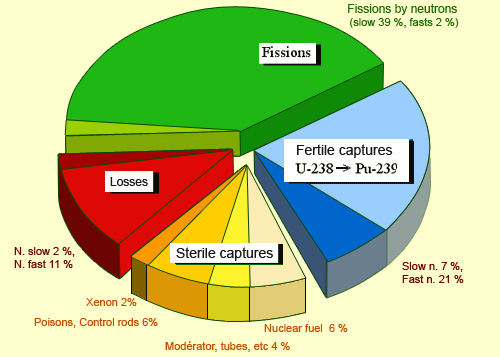
Distribution of slow and fast neutrons in a PWR
This repartition of neutrons in a pressurised water reactor shows the different roles played by slow and fast neutrons. Slow neutrons are responsible for most of nuclear fission and therefore help sustain the chain reactions. Fast neutrons, on the other hand, play a small role in fission but can transform nuclei of uranium 238 into fissile plutonium 239. Other neutrons are lost when they escape from the reactor or are captured by ‘sterile’ nuclei. Among these, some can poison the performance of the reactor, others are used for the control of the chain reaction (control rods).
© IN2P3
French pressurised water reactors work with uranium fuel containing 3.5% fissile uranium 235. For the chain reaction to be maintained, the neutron loss must be significantly reduced in the remaining 96.5% of the fuel and in the other non fissile materials in and around the core of the reactor.
The fission probability varies by several order of magnitude with the energy of the captured.neutron. For uranium-235, it is maximal with neutrons of very low energy, called thermal neutrons. Neutrons must be slowed down to low energies as soon as possible, to avoid being captured on their way by other nuclei and therefore lost. One should slow down to a very low energy, so that the proportion of neutrons that fission uranium-235 is the most favorable. To this end, the fissile material is merged with a medium, referred to as the moderator, effective in slowing neutrons without capturing them (such as water or graphite).
Thanks to this moderator, the average number of neutrons available for a secondary fission released (a property known as criticality) can be kept exactly at the safe value of 1. PWR reactors are for example designed in such a way that an excursion of criticality above that may leads to an explosive process is immediately and naturally corrected.
Other articles on the subject « Nuclear Fission »
200 millions electronvolts !
A huge amount of energy at the atomic scale The fission of a uranium or plutonium nucleus liberat[...]
Fissile nuclei
Uranium 235 and 233, plutonium 239 The few fissile nuclei found in nature belong to heavy atoms, [...]
Fission fragment
Chronicle of the life of a fission fragment … The fission of a nucleus into two fragments i[...]
Fission products
The ashes of nuclear fission Fission products are the remains of a heavy uranium or plutonium nuc[...]
Plutonium-239 formation
Transforming a fertile nucleus into a fissile nucleus Uranium-238 accounts for more than 95% of t[...]
Short-lived Fission Products
Most fission products vanish rather rapidly The vast majority of the radioactive fission products[...]
Long-lived Fission Products
A handful of tough but little radioactive isotopes … The more a nucleus is long-lived, the [...]
Actinides
A class of very heavy radioactive nuclei Alongside the fission products found in the core of nucl[...]
Plutonium Isotopes
Plutonium-239 but also 238, 240, 241, 242 … Plutonium isotopes are produced by neutron capt[...]
Minor Actinides
Neptunium, americium and curium Minor actinides constitute a very small minority of high activity[...]