Waste radioactivity decrease
A natural but slow decay process
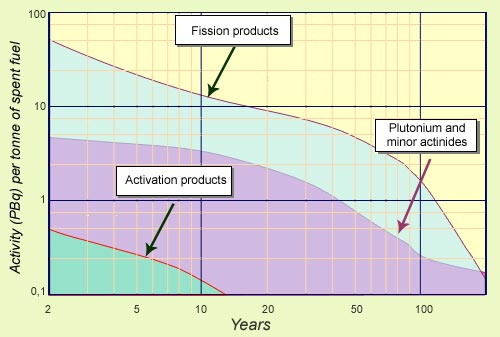
During the first century…
Irradiated fuel removed from nuclear reactors is still in its early years of radioactive decay. Activity levels remain very high. Fission products are the most highly radioactive, followed by plutonium and minor actinides. Radioactivity from fission products is overtaken after 200 years by that from plutonium, after decaying by two orders of magnitude. Activity generated by neutron activation of fuel cladding and end caps is at a far lower level.
© IN2P3
As natural radioactivity progressively decays, the radioactivity emitted by a radioactive waste package decreases over time. Although glaringly obvious to physicists and engineers, the layman is often unaware of this fundamental fact. There is a popular belief that a waste package will be nearly as radioactive in 1,000 years as it is today! Despite it being a law of nature, too few people are aware that radioactivity decays.
Decay occurs slowly but surely. According to a study by the Finnish nuclear safety authority, upon removal from a reactor, spent fuel is 4 million times more radioactive than the natural uranium from which it was made. One year later, this factor has fallen to 60,000. After 500 years, the fuel is still 100 times more radioactive. It takes around 200,000 for the radioactivity from the waste package to match that of natural uranium.
Although this decrease is spectacular when measured in centuries and millennia, it appears very slow on human timescales! As the initial activity is very high, it will be a long time before the risk becomes negligible. The overall evolution is made more complicated by the variety of radioactive elements contained in the fuel. Each element decays according to its own half-life yielding daughter products that in some cases are also radioactive.
yielding daughter products that in some cases are also radioactive.
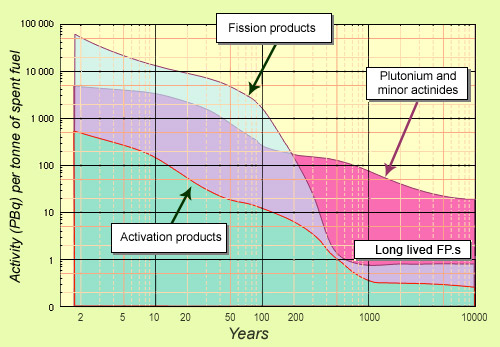
Spent PWR fuel activity from 10 to 10,000 years
This diagram compares the activity of fission products and actinides (plutonium and minor actinides) with that of activation products (cladding, package ends). Fission products are initially predominant, but are overtaken after around 200 years by plutonium and minor actinides, which decay more slowly. The decline in fission product activity tails off after 600 years, due to the presence of residual long-lived elements. Logarithmic scales (with powers of 10) must be used to represent the large changes in activity and time.
© IN2P3
Around 10% of fission products have a radioactive half-life longer than 30 years. Some have half-lives of thousands or even millions of years. It should be noted that elements with very long half-lives disintegrate very slowly, and their radioactivity is correspondingly lower.
Fission products decay the most rapidly. The activity of such elements declines by a factor of 7 over 10 years, by 60 over 100 years, and by tens of thousands within 500 years. Caesium-137 and strontium-90 make the biggest contribution during the first hundred years or so. When these intermediate-lived fission products disappear, a few long-lived fission products (iodine-129, caesium-135 and technetium-99) remain. Note that activation products systematically represent only a small proportion of total radioactivity.
Plutonium and minor actinides, which are heavy nuclei, generally have long half-lives, as do their daughter products. Their radioactivity decays much more slowly. Over the very long term, the activity of spent fuel is largely determined by plutonium – if it has not been removed – and minor actinides. This explains why such importance is given to these products. It also explains the benefit of removing them from waste, if they can be destroyed by transmutation.
The presence of residual long-lived radioelements causes the rate of decay observed during the initial centuries to slow after around 500 years. By this point, the fuel’s activity has already fallen by a factor of 40,000 since it was removed from the reactor. This residual amount is the focus of current research that seeks to further reduce it at millennial time-scales.
Other articles on the subject « Spent nuclear fuel »
Reactor unloading
Fuel unloading and initial pool storage of spent fuel The fuel load in a conventional pressurised[...]
Spent fuel Burn-up
Irradiation rate and energy supplied by fuel The energy produced by a nuclear power plant is prop[...]
Spent fuel composition
Key figures on spent fuel composition The composition of irradiated fuel removed from a reactor c[...]
Spent fuel decay heat
Significant, slow-to-decline heat release The heat released by radioactive disintegration is a ke[...]
Spent fuel radiotoxicity
A risk indicator more pessimistic than radioactive activity Radioactivity is an imperfect descrip[...]