Radioactivity Trojan Horse…
Radioactive atoms have two ways of sneaking past our body natural defences: ingestion and inhalation. Is this an atomic Trojan war? By hiding among the food we eat and the air we breathe, these atoms can enter our body in the same way as Greek soldiers, concealed inside Ulysses’ wooden horse, were able to penetrate the walls of Troy.
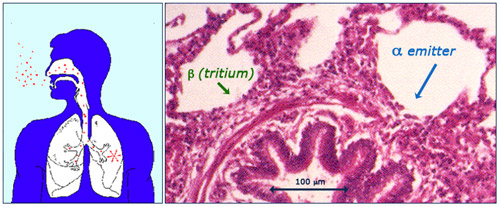
Irradiation of the lungs via inhalation
Radon descendants are responsible for a natural source of internal exposure. The lungs are affected when aerosols carrying these radioactive descendants for the radon gas are inhaled. Once deposited on the surface of the lungs, the radioelements emit alpha rays which can penetrate deep enough to reach the cells of the bronchioles sensitive to cancers. The beta rays emitted by tritium (a radioactive isotope of hydrogen produced by cosmic rays) can also reach the lungs, but deposit far less energy.
IN2P3/Source K.G.Gerber
Alpha and beta rays emitted by external sources are usually absorbed well before they reach our bodies. When a source of beta radiation is placed very close to the skin, however, a large dose of radiation can sometimes be absorbed.
External sources normally resort to bombarding us with their equivalent of long-distance missiles: gamma rays. Once the sources enter our bodies, however, the more effective short-range arsenal of alpha and beta particles can be deployed.
These particles deposit a large amount of energy, at the atomic scale, in the immediate surroundings of the atom which emitted them. Alpha particles in particular deposit a highly concentrated dose of radiation along a fraction of a millimetre. A similar behaviour is also observed on a lesser degree for beta particles, whose trajectories through matter are much longer than those of alphas. The amounts of energy both forms of radiation deposit in the body can cause damage to living cells and to their DNA.
A large number of factors influence the dose absorbed by the body following an internal exposure: the type of radiation involved, the chemical form of the radioactive atom, the spread of radiation throughout the body and naturally the exposure time. The chemical structure of the atom (whether it is free or attached to a molecule) is very important as it determines its journey through the body. As radioactive isotopes are chemically equivalent to their stable isotopes, they behave in exactly the same way once inside the body. Tritium will follow the path of hydrogen atoms, carbon 14 will behave as other carbon atoms, and iodine 131 will reach the thyroid gland along with all other atoms of iodine. When a radioactive atom is attached to a molecule, it is usually the chemical reactivity of this molecule which determines the atom distribution.
Once inside the body, radioactive elements normally enter the circulatory system and thence spread around the body. Some elements attach themselves to specific organs referred to as target organs. Concentrations of iodine 131 can be found in the thyroid, and certain alpha emitters target the lungs, the bones and the kidneys. On the other hand, certain radioactive isotopes such as cesium spread throughout the entire body muscular mass. A large fraction of this cesium leaves the body via natural processes, exposing the digestive tract and especially the colon. For instance, half of the cesium 137 ingested by an adult will have disappeared after 150 days – much sooner that would be expected from the isotope radioactive half-life of 30 years.
Radiotoxicity measures the radioactive toxicity of a substance once it is ingested or inhaled. The figure takes into account the energy deposited by the radiation, the atom chemical structure, the age of the exposed individual, the sensitivity of the affected organs and the length of time over which the exposure takes place. The higher an element radiotoxicity, the greater the potential of its ingestion. The received dose is calculated with the help of a coefficient known as the ‘effective dose per unit intake’ (d.p.u.i), expressed in Sieverts per Becquerel (Sv/Bq)
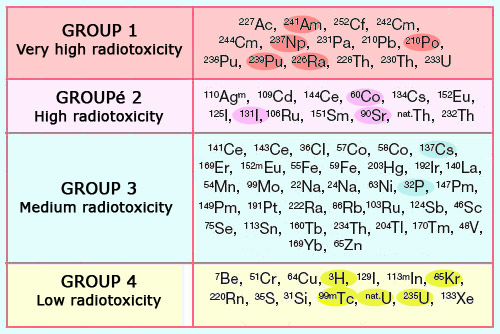
Classification according to radiotoxicity
The radioactive isotopes are classified into 4 groups according to their toxicity. The first group consists of alpha emitters: plutonium, neptunium, americium, curium as well as the natural polonium 210 and lead 210 isotopes. Among the beta emitters, the radioactive isotopes of iodine which affect the thyroid and strontium 90 (which attaches itself to bones) have a high toxicity, whereas caesium 137 (which spreads among the muscle mass) is less dangerous. At the lowest end of the scale one finds tritium (whose energy deposit is particularly low), krypton 85 (a noble gas which occurs as a by-product in nuclear industry) and metastable technetium 99 (frequently used in medical scans).
© IN2P3/ICRP
The most radiotoxic elements are the alpha emitters, which include elements like plutonium, the minor actinides produced in nuclear reactors, and isotopes resulting from the natural decay of uranium (radium is the best-known element in this last group). Far behind in terms of toxicity are the emitters of beta radiation, which include carbon 14, potassium 40 and tritium, as well as fission products created in nuclear reactors. The dose factor of tritium is especially low. Among the most radiotoxic beta emitters are the radioactive isotopes of iodine, whose concentration in the thyroid gland have had serious health consequences near Chernobyl.
The natural internal exposure resulting from carbon 14 and potassium 40 is practically harmless, owing to these isotopes’ low toxicity and inoffensive behaviour in the body. With the notable exception of radon, naturally-occurring alpha emitters also do little damage. The decay of this gaseous offspring of uranium can produce radioactive isotopes of bismuth, polonium and lead, which once inhaled attach themselves to the lungs. As a result it is the inhalation of radon which is the principal source of natural internal exposure.
Internal exposure is also used in a variety of medical techniques where patients are injected with radiopharmaceutical products in order to attack tumours and cure certain diseases.