What doses when swallowing or inhaling radioactive atoms ?
How to evaluate the doses resulting from ingestion or inhalation of radioactive substances ? How to translate activities measured by detectors – but difficult to understand – to effective doses that are of concern for the man in the street? In other words, how to convert becquerels into millisieverts
into millisieverts ?
?
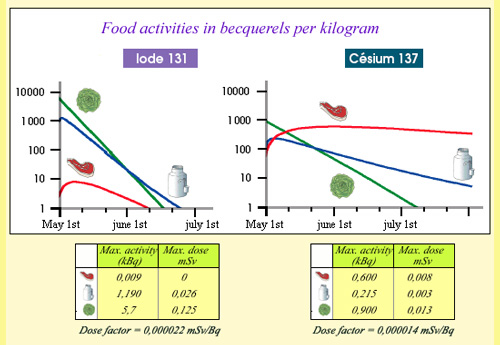
From activities to committed doses …
Example of committed doses due to the ingestion of contaminated food near Chernobyl. The curves show the evolution of the activity of iodine 131 and cesium 137, during the three months following the accident, for one kilogram of meat, of salad, a litre of milk coming from the most contaminated areas. Activities were considered at their maximum. The maximum doses were obtained by multiplying these activities by the iodine 131 and caesium 137 doses factors. In the most hazardous case of salads, the ingestion of 5.6 kBq resulted in a dose of 0.125 mSv, equivalent to 15 days of exposure to natural radioactivity in France.
© IRSN
When radioactive atoms are ingested, the resulting effective dose is calculated through ‘dose conversion factor. A dose factor per ingestion is considered for each radioactive species ingested. Dose factors allows to compare the nocivity of radioactive elements (radioelements), for an equal ingested activity.
The effective dose resulting from the ingestion of a certain amount of a radioactive substance would be calculated from the radioactive activities of the radioelements contained therein. For each radioelement involved, its dose factor is first multiplied by the activity (number of becquerels) ingested. Then, the effective dose is obtained by summing all the radioelements involved.
There are also tables of inhalation dose factors, whose use is less straightforward because these factors depend on the size of the aerosols which carry the radioactive atoms.
The relevant effective dose obtained is a committed dose, i.e. a dose evaluated for a person’s lifetime. This evaluation depends on the method of ingestion and the path taken by the radiation through a person’s body.
One calculates “equivalent” and “effective” committed doses resulting from ingestion or inhalation of a radioactive substance. One has to estimate, nearly on the scale of a human life, the final dose that will be suffered by a tissue (or organ) or by the whole body. The assumed time span is 50 years for adults and 70 years for children in order to reflect the phasing out of radioactive substances or their non-elimination for some of them.
Alpha emitters (such as uranium, plutonium, and the actinides produced by nuclear reactors) are far more dangerous in this regard than beta emitters (such as the products of nuclear fission). We can also see that the inhalation of alpha emitters (and their consequent presence in the lungs) is several tens of times more hazardous than their ingestion.
The paths of radioelements in the human body differ according to their chemical properties. Radioactive atoms will undergo the same metabolic processes as their neighbours in the periodic table. This is why radium can be found alongside calcium in the bones, and why iodine-131 as well as its stable isotopes both target the thyroid gland.
The thyroid gland plays a particularly important role before adulthood, and as a result children and adolescents are far more sensitive than adults to radioactive iodine 131. A toddler will be over ten times more sensitive to radioactive iodine that makes its way to the thyroid.
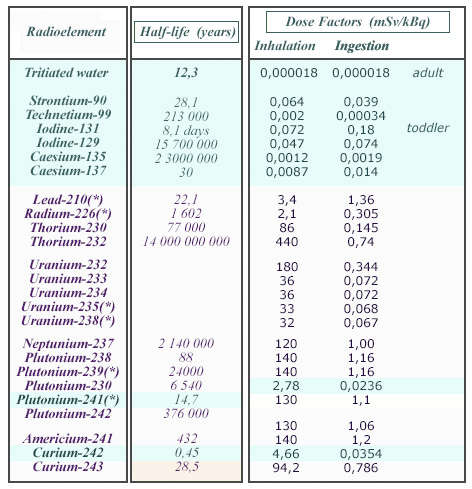
Table of dose factors
These inhalation and ingestion dose factors shows large variations between radioelements. Since all dose factors expressed in Sv/Bq are very small, they have been given in mSv/kBq. The beta emitters (tritium, fission products etc) are displayed on a blue background. One remarks the much higher radiotoxicities of alpha emitters, especially inhalation radiotoxicities. The symbol (*) indicates when radioactive descendants are taken into account in calculating the doses factors.
© Source: Annex of CNE 2003 report
In order to evaluate dose factors the most accurately possible, radiobiologists use models which take into account the type and strength of radiation involved, as well as the way in which energy is deposited in the body and the age of the recipient. These models, though inevitably imperfect, make use of the most up-to-date research in the field.
Doses factors tables are then published and updated annually to include the more recent data. An example of these tables can be found in the publication 119 of the ICRP (Annexes F and G, pages 71 and 87)
The values of dose factors, which convert ‘activities’ in becquerels to ‘doses’ in sieverts, are very tiny because the Sievert (Sv) is a large unit and the Becquerel(Bq) a very small one. The largest dose factors of heavy elements such as plutonium are around one ten-millionth of Bq/Sv, whereas light elements such as tritiated water have much smaller factors of closer to one hundred-billionth Bq/Sv.
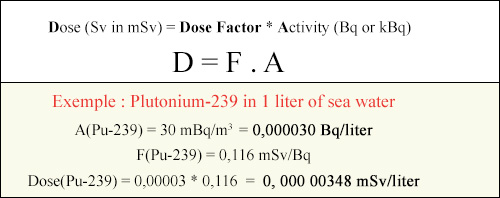
Dose calculation according to absorbed activity
Let us consider the exemple a diver swallowing a liter of seawater near the La Hague reprocessing plant. What dose would he be exposed due to traces of plutonium in this water? The plutonium activity measured by the IRSN due to discharges into the sea of radioactive effluents is very low: 30 mBq / m3 (i.e. 0.03 disintegration per cubic meter of water). Despite the high plutonium radiotoxicity, the dose absorbed by the diver would be 0.00000348 mSv, well below the legal limit of 1 mSv / year for accidental exposures.
© IN2P3
The above formula shows how one multiplies the dose factors (F) by the ingested activity (A) to evaluate the committed dose (D), and thus convert becquerels (Bq) to millisieverts (mSv). The example chosen is that of a measurement of the measured plutonium activity found in seawater near the french La Hague reprocessing plant. The measured activity is found to be 30 thousandths of becquerels (mBq) per cubic meter. What would be the dose taken by a diver if he dranks a liter of this seawater? This dose is obtained by multiplying the seawater activity to one liter by the plutonium dose factor extracted from the table.
Other articles on the subject « Radiation Effects »
Biological Effects
Living matter: both fragile and robust The amount of damage radiation can cause to living matter [...]
Probabilistic effects
The domain of low and average doses For weak or medium-strength doses, the effects are not as cle[...]
Deterministic effects
The domain of strong doses and severe, reproducible effects For high doses above a certain thresh[...]
Dose-effects Relationship
Can effects caused by low doses of radiations be predicted ? Even though we are constantly expose[...]
Dose Rate
Acute and chronic exposures The “effective dose” is not sufficient by itself to chara[...]
Hiroshima Nagasaki Survivors
An exces of cancers and leukaemia among survivors Our knowledge of the risks of cancers due to ra[...]
Low doses effects
Do low doses of radiation have any effect? Radioprotection experts commonly refer to doses below [...]
Linear No-threshold Model
A Precautionary Principle applied to radioactivity … Is it possible to predict the effects [...]
Radioactive Toxicity
A useful indicator but to be used appropriately … The danger presented by a radioactive sub[...]