Radioactive substances older than the Earth

The Crab nebula
The Crab nebula is all that remains of an explosion which took place 6,000 years ago and was observed on Earth in 1054 by Chinese astronomers and Navajo Indians. It is the explosions of such supernovae that can cause atoms to capture more neutrons and form heavier elements such as platinum, gold or uranium. These are then dispersed by the explosion into the furthest reaches of the galactic space.
IN2P3/NEPAL
The principal source of natural radiations on Earth is the presence of three radioactive nuclei in our planet crust: thorium 232, uranium 235 and uranium 238. Their extremely long half-lives (of the order of billions of years) mean that none of these substances will be disappearing any time soon, and as a result they form an integral part of our terrestrial environment.
These three radioactive nuclei, the heaviest found in nature, have been present in the Universe since the early clouds of interstellar dust which combined and solidified to form the planets and the stars. These clouds of dust were the products of supernova explosions, spectacular events which mark the death of very large stars and release enough energy to produce nuclei heavier than those of lead. Significant quantities of these three radioactive nuclei also went into the formation of the Earth nearly 4.5 billion years ago.
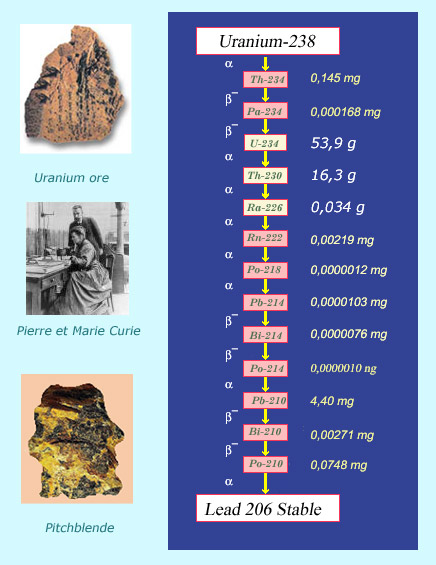
Descendants of uranium 238
The radioactive offsprings of uranium and thorium contribute to natural radioactivity. Traces of uranium descendants can be found in uranium ores, with 0.34 grams of radium and 0.0000012 milligrams of polonium found in every ton of uranium. The family tree of uranium 238 ends with lead 206, which is a stable and non-radioactive, as opposed to the preceding thirteen radioisotopes, all of which have equal activities. It was by observing that two different uranium minerals, pitchblende and chalcolite, had higher activities than uranium itself, that Pierre and Marie Curie discovered the tiny quantities of polonium and radium they contained.
© IN2P3
Thorium 232, uranium 235 and uranium 238 are the ancesters, the genitors of three distinct radioactive family trees. These nuclei decays to produce other radioactive nuclei with shorter half-lives, a continuing process which for the sake of clarity is often compared to human genealogy. This ‘radioactive descendance‘ explains why traces of radium can be found alongside uranium inside blocks of granite. Radium has a half-life of only 1600 years, and would have completely disappeared from the face of the Earth were it not for the fact that it is constantly regenerated by its radioactive ancestor, uranium 238.
Telluric radiations, principally made up of gamma rays emitted by the atoms of thorium, uranium and their descendants found in rocks, are a major source of external exposure for living matter. The intensity of the radiations emerging from the rocks is measured at a height of one metre from the ground, and is then used to calculate the effective dose that would be absorbed over the course of a year. The resulting value is then often expressed in milligrays (or mGy) per year, with the global average being estimated at around 0.4 mGy per year.
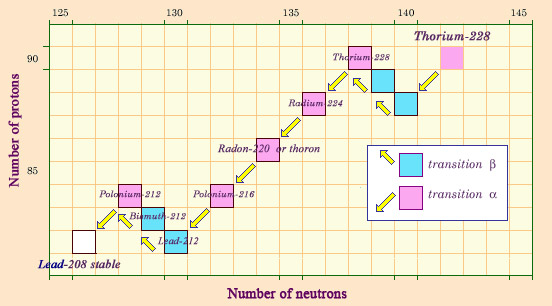
Descendants of thorium
Naturally occurring thorium 232 is also the patriarch of a chain of radioactive elements which decay into each other, ending with the formation of a stable isotope of lead, lead 208. The genealogy of thorium also contains a gaseous element, radon 220, historically known as thoron. This form of radon is less dangerous than that released by uranium, however, as the half-life of radon 220 is only 55 seconds, too short for it to be able to escape from the rock.
© IN2P3
In France, this intensity averages to around 0.7 mGy per year although the granite-rich areas of Brittany can exceed 2.5 mGy per year. These numbers are still far lower than the 50 mGy per year observed on some black sand beaches in Brazil or in Southern India, caused by the presence of the mineral monazite, 10% of which is made up of radioactive thorium.
On top of the telluric radiations we have to consider the contribution of radon as well as its radioactive progeny. Radon-222 which, belongs to the uranium family, is the principal natural source of exposure to radiation, with an average of 1 millisievert (mSv) per year in France. Radon contrarily to the other descendants of uranium-238 is gaseous. Its half-life is 3,8 days. When radium decays to radon, the radon atom can escape from the rocks and dissipate in the atmosphere. As a ‘noble’ or unreactive gas it cannot attach itself to the human body but its adioactive descendants do have the ability to do so. Radon exposure varies also greatly from place to place. Modern regulations aim to provide good ventilation to insure radioprotection against the effects of radon.
NEXT : Cosmogenic Radioelements
Other articles on the subject « Natural Radioactivity »
Natural Origins
From vestiges of earth formation to cosmic rays Despite having evolved under constant exposure to[...]
Cosmogenic Radioelements
Formation of radioactive atoms from cosmic rays The Earth is constantly being bombarded by ‘[...]
Natural Exposure
A chronic but benign exposure All exposure to radioactivity, whether natural or artificial, is me[...]
Ground Radioactivity
Telluric Exposure : Radiation which emanates from rocks The Earth crust contains a number of radi[...]
Human Internal Exposure
Our bodies are also… slightly radioactive All through our lives, we inhale and ingest radio[...]
Radioactivity in food
In our glasses and on our plates … The very acts of breathing and walking around make it im[...]