Formation of radioactive atoms from cosmic rays

Cosmic Rays collisions
A primary cosmic particle entering the Earth’s atmosphere generates a shower of secondary particles. Collisions with oxygen or nitrogen nuclei create radioelements such as radiocarbon or tritium. Some secondary particles will reach the ground and hundreds of them pass through our bodies every second. This cosmic bombardment makes up a part of the natural radioactivity we are constantly exposed to.
© INFN/IN2P3
The Earth is constantly being bombarded by ‘cosmic rays‘, highly energetic particles coming from the depths of space. These particles (known as primary) can be either electrically charged – with protons making up 86% of charged particles, alphas making up 13% and the remainder consisting of heavy nuclei – or neutral, in which case they primarily consist of photons and neutrinos.
The energy of these cosmic rays varies enormously, as the variable magnetic field of the stars is capable of accelerating certain charged particles to energies of over 1 billion electronvolts and much more. When these particles then approach the Earth they are deflected by our planet magnetic field, which serves as a giant shield against these extraterrestrial bombardments. This shield is weakest, however, near the magnetic poles, and it is the cosmic rays which manage to penetrate that are the cause of the aurora borealis.
Upon entering the higher layers of the atmosphere the cosmic rays start colliding with nuclei, causing an explosion of pieces of subatomic shrapnel which in turn crash into other atoms. This chain reaction of collisions produces a host of ‘secondary‘ particles, some of which will reach the Earth surface like muons (particles similar to electrons, but 200 times heavier and highly unstable) and neutrinos.
The majority of these neutrinos are produced by the Sun, though others come from further in the galaxy. Some neutrinos are produced in the upper atmosphere from the collisions of other cosmic particles. Billions of these ghostly particles pass through our bodies every second, causing no damage as they practically never interact with matter.
Muons interact little and do not produce radioactivity. Neutrinos, which are capable of passing unhindered through an object as wide as a star, very rarely produce any secondary particles.
During the development of the shower of particles that follows the initial collision, many of the atomic nuclei in the atmosphere that serve as a target could be broken, protons and neutrons emitted. These neutrons can penetrate into the nuclei of other atoms, mainly oxygen and nitrogen, to make them radioactive. These radioactive nuclei, byproducts of cosmic radiations, are called cosmogenic.
One of these neutrons entering a nucleus of nitrogen from the air triggers the ejection of a proton or a proton bound to two neutrons (a tritium nucleus). In the first case, the nitrogen is transformed into radioactive carbon-14; in the second case the nitrogen is transformed into stable carbon-12 but is accompanied by radioactive tritium.
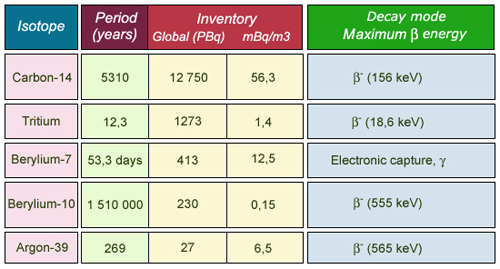
Main cosmogenic nuclei
Carbon-14 and tritium are the main radioactive elements generated by cosmic rays. The inventory represents the total activity in the atmosphere estimated at the scale of the Earth (1 PBQ equals 1 million giga-bequerel). Some of the carbon-14 is absorbed by plants and for tritium in water. The order of magnitude of activities are of millibecquerels per cubic meter of air.
©IN2P3 (Source Cours K.Gerber)
Among the “cosmogenic” nuclei created in these atmospheric collisions, some are radioactive: notably carbon 14, tritium, beryllium 7 and beryllium 10. Some of these decay rapidly whereas others, such as carbon 14 (created from nitrogen found in the air) live for longer. Molecules of this carbon 14 are therefore often absorbed by plants alongside ordinary carbon, meaning that all living matter contains traces of this radioisotope.
Other articles on the subject « Natural Radioactivity »
Natural Origins
From vestiges of earth formation to cosmic rays Despite having evolved under constant exposure to[...]
Uranium and thorium origins
Radioactive substances older than the Earth The principal source of natural radiations on Earth i[...]
Natural Exposure
A chronic but benign exposure All exposure to radioactivity, whether natural or artificial, is me[...]
Ground Radioactivity
Telluric Exposure : Radiation which emanates from rocks The Earth crust contains a number of radi[...]
Human Internal Exposure
Our bodies are also… slightly radioactive All through our lives, we inhale and ingest radio[...]
Radioactivity in food
In our glasses and on our plates … The very acts of breathing and walking around make it im[...]