After an accident : what impact on food and man ?
A large amount of radioactivity was dispersed into the atmosphere during American and Soviet atmospheric nuclear weapons tests of the years 1950-1960, the Chernobyl accident in 1986 and to a lesser extent that of Fukushima in 2011.
A small part of the dispersed radioactive atoms has contaminated the food chain and eventually reached our bodies and exposed us to radiation. To what extent is food contaminated ? What is the scale of the exposure? What are their dangers?
Putting aside the short lived radioactive atoms – such as Iodine-131 – whose nuclear activity decrease quickly, the two main elements to consider for both the amount released and the life duration are Caesium-137 and Strontium-90 (their half-lives are around 30 years). Caesium-137 is the most studied of these two radioelements because it emits gamma radiation which makes easy identifying its presence (NB : Strontium does not emit gamma and is more difficult to study. it would be less mobile and follows a similar pattern) .
Although being moderately radiotoxic (as it mainly remains in muscular tissues), Cesium raises legitimate concerns among the public, because of its lifespan. It takes a long time – 30 years – for its nuclear activity to be halved !
How many Caesium atoms will end up in our food among all those which were dispersed and are slowly sinking into the ground? How do they bind to our human body? How long do they stay?
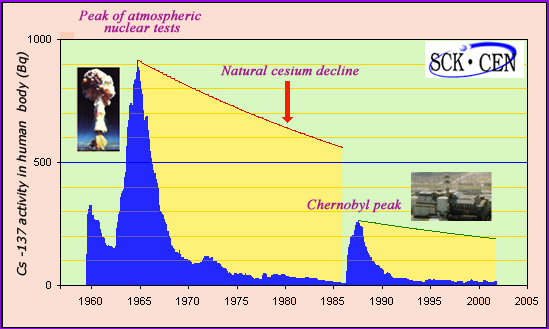
Caesium-137 evolution in human body
The activity of caesium 137 in the human body has been continuously monitored by the Mol laboratory in Northern Belgium for the past half century. Despite being far removed from the sites of nuclear tests and of the Chernobyl accident, one can see clearly the peaks in activity caused by the tests and, later on, the peak caused by Chernobyl. In both cases, the decay of caesium 137 found in the body is much quicker than the natural radioactive decay with its period of 30 years. Even though caesium slowly enters the soil, the rapid decay of the activity in humans has made it unnecessary for the Belgians to decontaminate their grounds.
© SCK-GEN (Source Jean Louis Genicot)
A study carried out in Belgium provided answers. Mol laboratory, in Belgian Flanders, measured each year for nearly half a century the nuclear activity of Caesium-137 in the human body. The laboratory is located away from nuclear test sites used in 1950-60 and from Chernobyl. These measures give a good idea of the exposure experienced during this period by the vast majority of the human population living away from these sites.
The activity of Caesium-137 is to be compared with the 8,000 becquerels corresponding to the human body natural activity. This activity comes mainly from carbon-14 and potassium-40. At the peak of atmospheric nuclear tests, this activity peaked at just over one tenth of the human body natural activity (900 Bq), falling soon after at a low level. When the Chernobyl accident occurred in 1986, it rose again to 3.5% of the natural activity (270 Bq) to fall after 3 to 4 years at a low level in the range of 20 to 40 becquerels.
Why is the activity absorbed by the human body decreasing faster than the nuclear activity of caesium? The answer lies in the fact that the presence of radioactive atoms is linked with the ingestion of contaminated food, in particular in farm products such as meat, milk, fruits and vegetables.
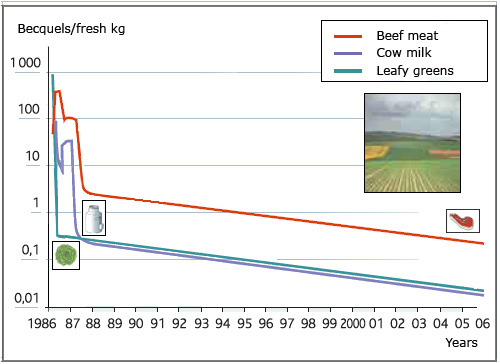
Natural decrease of radioelements in food
In 1987, one year after Chernobyl, the contamination of agricultural production came from the root uptake of radioactive cesium atoms present in the ground rather than by deposits on the leaves and grass as it was in the weeks after the accident. The Caesium transfer to plants and food became much lower and declined steadily over the years. Twenty years later, the contamination of agricultural products was 10-30 times smaller than in 1987 and 1000 to 10 000 times lower than that it was at the time of deposit in early May 1986.
© Source IRSN
The decrease in the presence of caesium in the human body reflects the natural decrease in contamination of food, as observed in the analyses of the Institute for Radiological Protection and Nuclear Safety ( IRSN ) conducted in France after the Chernobyl accident. In the year 1986 the main exposures – three quarters – are due to the contamination of food, followed by deposits on the ground and inhalation. The passage of the cloud (gamma rays) is marginal. Since 1987, the contamination of the food chain has steadily declined. Twenty years later, the annual internal doses are 50 to 100 times lower.
In 1986, during the accident, the dispersed Cesium deposited on the grass, on the leaves. It was spring ! Cows grazed this contaminated grass. Salads and vegetables with large leaves were affected. A year later, a new vegetation appeared and replaced the previous one. Since 1987, much less contamination has entered food. Contamination of agricultural products is no longer coming from the radioactive deposits on leaves and grass, but from the root uptake of cesium134 and 137 on the ground, which is a process one order of magnitude less effective.
In 2006, the annual dose to the French population as a result of contamination inherited from the Chernobyl accident was estimated to 0.010 mSv, which is the equivalent of a little more than a day of natural radioactivity.
Other articles on the subject « Accidental Releases »
Atomic bombs legacy
A legacy of the cold war The residue of radioactive contamination which still endures has its ori[...]
Atmospheric Releases
Atmospheric radioactive pollution after a major accident During a major accident, radioactive mat[...]
Risks of ground deposits
Two deposition modes: dry deposits and wet deposits In ordinary life, apart from the vicinity of [...]
Exposition modes (accident)
What are the exposition pathways after a major accident ? What is the impact on man of a major re[...]