Radon and its descendants
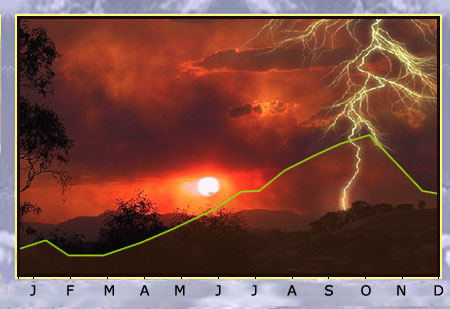
Radon in the environment
The concentration of radon in air depends on the type of soil and meteorological conditions such as air pressure, humidity, temperature, precipitation and wind speed. It has been observed that the concentration of radon in air increases significantly after a thunderstorm. The graph above shows the variation in radon concentration over time for the Massif Central region in France. It can be seen that February is the month with the lowest concentration of radon, and October with the highest.
© IRSN
First discovered in 1899, radon is a colourless and odourless naturally occurring gas. It is a noble gas (and therefore chemically inert) but can be dissolved in water. Generally, radon quickly spreads into the atmosphere once it leaves the ground and enters the air.
Located between uranium and lead in Mendeleev’s periodic table, radon is referred to as a very heavy atom. It is denser than any other natural gas, and as a result tends to accumulate in closed spaces such as buildings.
Radon travels upwards in the soil until it reaches the atmosphere, where it can be found in minutes quantities. Its concentration varies greatly throughout the air, and has been found to change with geological and meteorological conditions. The story of radon would be unknown to anyone outside chemical circles, were it not for the fact that its radioactivity makes it the principal source of radiation occurring in nature. For those living in France, the inhalation of radon and its descendants makes up a third of their exposure to radiation.
Radon is a radioactive descendant of the uranium, present in the Earth crust, and as a result can be found everywhere on the Earth surface. Its radioactive half-life is short – only 3.82 days – but the supply of radon is constantly regenerated by the disintegration of radium-226 , itself a descendant of uranium 238. Even though uranium is a rare element in nature, it can be found in most rocks and particularly in granite and volcanic soil.
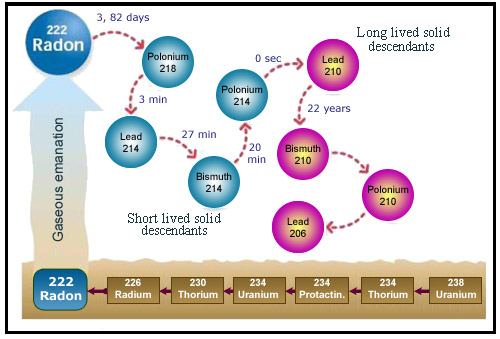
The descendants of radon
An atom of radon successively decays into atoms of polonium 218, lead 214, bismuth 214 and polonium 214, all four of which can be inhaled. Owing to its very short half-life (16ms) polonium 214 does not deposit directly into the lungs, but the other three can enter the body and continue the decay process until they reach lead 210. This isotope of lead is itself radioactive, but can be viewed as the final product of radon decay. It has a half-life of 22 years, but its concentration is very low and its activity negligible.
© IRES/IN2P3
The danger posed by radon (which is itself an inert gas) is primarily due to the alpha radiation emitted by its descendants – which are neither gaseous nor inert. The decay of radon into polonium 218 is followed by further alpha decay into lead 214. Lead 214 emits then beta electrons to transform into bismuth 214 and polonium 214, which produces lead 210 through the emission of a third alpha particle. This series of transformations takes place in a time span roughly equal to an hour.
This time span allows the three first descendants (polonium 218, lead 214 and bismuth 214) to attach themselves to microscopic particles – aerosols -which, if inhaled, may fix themselves to cells within the lungs. Polonium 214, the penultimate descendant, does not have enough time to be inhaled by itself into the lungs as its half-life is of only 1.6 hundredths of a second.
The alpha ray emitters which fix themselves to the inside of the body are dangerous. The alpha rays they emit are much more damaging to living tissue than beta radiations, as they deposit their energy in a far more concentrated manner. If we associate this energy with toxicity then polonium 218 is the most toxic, as it emits an alpha particle of 6.0 MeV followed by a second alpha particle with an energy of 7.69 MeV when it transforms into polonium 214. When the fixation in the body occurs after the polonium 218 decay, the alpha energy deposit is lower : the three following descendants only emit the 7.69 MeV alpha particle of polonium 214.