Amplify the traces of the passage of a microscopic particle …
The phenomenon of ionization enables easy detection of the passage of particles in matter. To ionize the particle must carry an electric charge or set in motion particles carrying an electrical charge.
While the charged particles interact continuously with the environment, the neutral particles interact through isolated events: a photon snatches an electron in an atom; a neutron collides with a nucleus or causes a nuclear reaction. This will be the secondary ionization due to the snatched electron, the recoiling nucleus or the products of nuclear reaction that signal the presence of the photon or neutron. The neutral particle delegates in some ways to secondary particles the cares of ionize.
For these reasons, a charged particle leaves a direct trace of its passage allowing detection. On the other hand a photon or a neutron may leave the detector without leaving a trace, then escaping observation.
Detect is first to identify the presence of a radiation, what a Geiger counter does, for example. Sophisticated detectors, used in laboratories or hospitals, go further in identifying its nature and by measuring its energy, its speed, and its position.
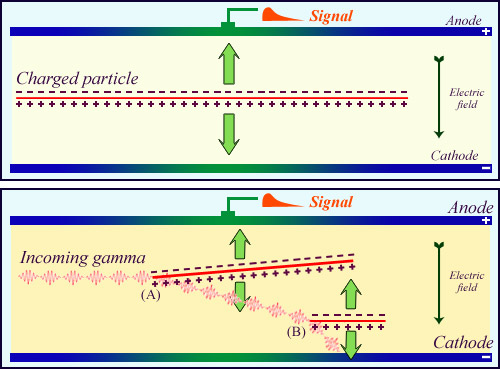
Amplification through ionization
Along its path, a charged particle (top) snatches thousands of electrons leaving behind as many positive atoms (ions). The presence of an electric field allows to collect all these positive and negative electric charges on an anode and a cathode. The collection of so many charges generates a measurable electrical signal. In the case of a neutral particle (below) the particle do not ionize directly. The gamma of the figure leave no trace, until it set in motion an electron (A), and later a second one (B) which will ionize. There is also a signal due to these secondary electrons.
© IN2P3
The detectors use the disruption caused by the passage of radiation and the manifestations of the return to equilibrium of the atoms affected by this passage. Ionized matter fells ill at ease and is trying to return to equilibrium. One attempts to observe this return to equilibrium, directly (for example, in scintillators) or after a delay by collecting the electric charges produced (ionization detectors).
Detection requires a double amplification. The first very important amplification is provided by the ionization process itself. It takes 30 electronvolts to snatch an electron from an atom of gas, and only 3 to snatch an electron from a silicon crystal. An alpha of 5 MeV tears 170,000 electrons in gas and 1.7 million in a silicon detector.
The energy deposited in the middle, although very important to the atomic scale, is still too small to be observed. It takes a second amplification provided by the detection system. For example, in a Geiger counter a wire set to a high voltage attracts electrons extracted by the ionization. When they approach the wire, they are strongly accelerated and snatch its turn other electrons. There is an avalanche of electrons, a phenomenon that generates an observable electrical signal.