Counting the decays of radioactive atoms
Radioactivity is an unrivaled instrument for exploring the environment and the living world, thanks to the extraordinary sensitivity of detection techniques.
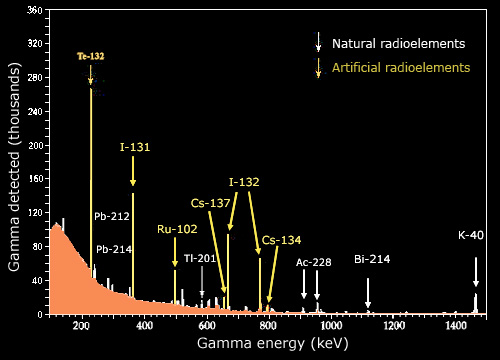
Sensitive detectors
The extreme sensitivity of radiation detectors is illustrated by the energy spectrum of gamma rays emitted in this sample by radioactive atoms. Modern germanium-based detectors capable of measuring accurately also the gamma energies, display the ‘characteristic rays’ emitted by different elements. Given the extremely high counts that are measured, one can observe the characteristic rays of elements present in very small amounts, such as caesium 134.
© IN2P3
We now have devices capable of detecting the individual decay of a nucleus. The possibility of observing such a tiny event is due to the combination of two factors.
– The radiations “electrifies” a large number of atoms along their paths, either directly in the case of alpha and beta rays or by setting in motion charged particles in the case of gamma rays. Hundreds of thousands of ionized atoms provide a first amplification.
– The effects of this primary ionization are then amplified by electronic devices having high gains. It becomes possible to collect a signal in a detector.
In addition to these two favorable factors, there is the extraordinarily large number of atoms present in matter. So 18 grams of water, the equivalent of a sip, contains 600 trillion trillion molecules. The Avogadro is so large, that even if the proportion of radioactive atoms is very low in a sample of matter, their number remains colossal. Detector counting rates, ie the observed numbers of decays per second, appears consequently always high.
The enormity of Avogadro’s number means that one can detect minute quantities of radioactive atoms, up to one part per million billion atoms. In comparison, a very toxic poison (like arsenic) require to be detected chemically proportions at best a billion times greater.
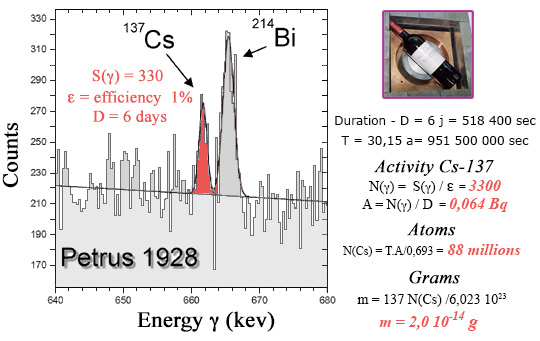
Amount of cesium-137 found in a false Pétrus 1928 grand cru ?
The measurement lasted 6 days. To calculate the cesium-137 activity, one should multiply the number of gamma detected by 100 to account for the detection efficiency of 1% and divide by 518,400 (number of seconds in 6 days). One gets 0.064 decay per second (becquerel). The number of atoms in the bottle is obtained by multiplying by the half-life of cesium-137 expressed in seconds (951 million) divided by 0.693 (the logarithm of 2). The apparently impressive results, 88 million cesium-137 atoms, corresponds to 0.2 millionth of a billionth of a gram in the bottle.
© CENBG/PRISNA CENBG/PRISNA
To illustrate the extreme sensitivity of radioactivity measurements, let’s take the example of the analysis carried out at the CENBG laboratory in Bordeaux of a bottle of Grand Cru which turned out to be a fake. Traces of cesium-137 were found in this bottle, a radioelement spread in the atmosphere during nuclear tests in the 1950s but which did not exist in 1928, the supposed vintage of this bottle sold as a Bordeaux Grand Cru Classé.
The presence of cesium-137 was demonstrated using an experimental device specializing in measurements of very low radioactivities. The bottle was placed in a lead shielded enclosure to reduce the gamma rays from natural radioactivity. Cesium-137 emits a gamma ray of 662 keV, with a very characteristic energy, which undoubtedly indicates its presence. During the 6 days that the measurement lasted, an excess of 330 counts in the vicinity of 662 keV was recorded, one count every 26 minutes! The measured activity was a few hundredths of a becquerel. The scam’s signature cesium-137 amount was 0.2 millionth of a billionth of a gram!

Césium et thons rouges
Californian researchers measured slightly higher levels of cesium-137 in muscle tissue from fifteen bluefin tuna caught off San Diego in August 2011, than in bluefin tuna caught before the Fukushima disaster. It is the presence of Cesium-134 that establishes a link with the Japanese accident. These results demonstrate the ability of large fish to migrate from Japan across the Pacific and the extraordinary sensitivity of detectors able to detect traces of radioactivity.
© Source : le Monde/AFP – 25/05/2012
A second example of the extreme sensitivity of the detection of radioactivity is that of the traces of cesium-134 and 137 found in the flesh of bluefin tuna caught in California one year after the 2011 Fukushima accident Like the Bordeaux laboratory, the Californian laboratory which carried out the analyzes was able to identify these traces of cesium. It is the level of radioactive activity that accounts for the importance of contamination, and not the fact of having detected radioelements.
Paradoxically, the results of this extraordinary sensitivity, which should reassure, are often interpreted with concern. We are able to detect radioactivity everywhere! Even radioactivity as innocent as that of our human bodies makes a detector rattle !